- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Pharmaceutical Spray Drying Fact Sheet
What is Pharmaceutical Spray Drying?
Pharmaceutical Spray drying is a simple yet incredibly flexible technology that can transform liquids into powders in seconds.
Manipulation of either the feed solution composition and/or the spray drying process parameters allows the creation of spray-dried powders with a range of both physical and aerodynamic properties.
Whilst formulation and process development for pharmaceutical spray drying can be readily undertaken at a small, even milligram scale if the drug is in short supply, the process is highly reproducible and scalable, with established models allowing straightforward scale up to produce kilograms of material for toxicology and clinical studies.
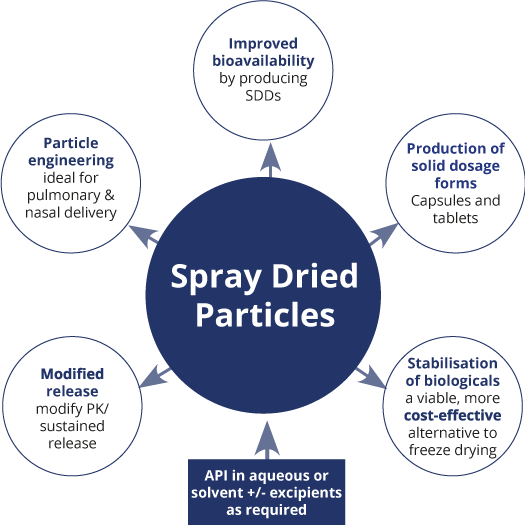
There are several key features of the spray drying process that can be manipulated to enhance the formulation and delivery of small molecules and biologics. In many cases, several of these features can be combined and as a result the target product performance profile can subsequently be achieved.
View our spray drying fact sheet.

The ability to ‘engineer’ the physical properties of the powders produced has made spray drying one of the fastest growing and most versatile processing technologies in the pharma and biotech industries.
We're here to help
If you’re looking to work with a CDMO with extensive experience in overcoming solubility challenges through spray drying, then we’re here to help.
Speak to our team to discuss your requirements.
This ability to ‘engineer’ the physical properties of the powders produced has made spray drying one of the fastest growing and most versatile processing technologies in the pharma and biotech industries. This technology is now being used to manufacture a wide range of dosage forms, with actives including small molecule APIs through to large, complex biologics.
The Spray Drying Process
Spray drying is a simple yet incredibly flexible technology that can transform liquids into powders in seconds. Manipulation of feed solution composition and process parameters allows the creation of spray-dried powders with a range of physical and aerodynamic properties.
Spray Drying Applications
Spray Drying at Upperton
Upperton is world-renowned for its’ spray drying expertise. We have a range of spray dryers available, from development dryers that can work with the smallest quantities (milligrams) of drug, to pilot-scale dryers that can produce kilogram quantities of powder in a single day of operation. These are available in our R&D and GMP facilities, meaning that processes can be seamlessly developed, scaled up and then transferred for clinical manufacture.
| Upperton Spray Dryers | ||||
|---|---|---|---|---|
| Spray Dryer (Number of Units) | Facility | Typical Batch Scale | Specialist Capabilities | Applications |
| Buchi B290 (8 units) | R&D | 100mg – 100g | Air/inert (N2) drying gas | Early formulation screening and development |
| ProCepT 4M8-TriX (3 units) | R&D and GMP | 100mg – 400g | 2-fluid and ultrasonic atomisation | Smaller scale GMP processes. Particle engineering for nasal and pulmonary delivery |
| GEA Niro SD Micro | GMP | 5g – 3kg | Air/inert (N2) drying gas | GMP manufacturing under nitrogen |
| GEA Niro MobileMinor (2 units) | R&D and GMP | 25g – 5kg | 2-fluid and pressure nozzles | Larger scale aqueous spray drying |
Whilst formulation and process development for spray drying can be readily undertaken at a small, even milligram scale if the drug is in short supply, the process is highly reproducible and scalable, with established models allowing straightforward scale up to produce kilograms of material for toxicology and clinical studies.
There are several key features of the spray drying process that can be manipulated to enhance the formulation and delivery of small molecules and biologics. In many cases, several of these features can be combined to achieve the target product performance profile. These can be summarised as follows:
| Spray Drying Process | Pharmaceutical Application |
|---|---|
| Spray drying a solution of API and excipients(s) to create a dry powder |
|
| Spray drying to create particles with defined size/aerodynamic properties |
|
| Modified solubility/release by co-spray drying with enteric polymers |
|