- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Choosing Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Accelerate drug development with an integrated CDMO partner.
Expertise to accelerate and de-risk your drug product journey.
Start your next project with Upperton.
As a leading UK-based CDMO our defining traits lie in our adaptability, nimbleness and expertise in oral, nasal and pulmonary dosage forms.
Through our science-led approach we align to your drug development needs from pre-clinical to late phase manufacture, across oral solid dosage forms, liquids, semi-solids, nasal and inhaled products.
Science-led expertise providing you with unparalleled project delivery.
Our team support you at each stage of your drug program lifecycle from pre-clinical to market.
Our CDMO services include formulation development, phase 1, phase 2 and phase 3 clinical supply, process scale-up and optimisation, quality control, analytical development and validation, and registration activities.

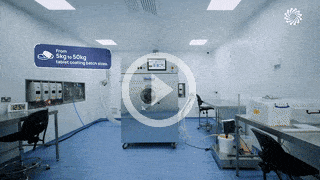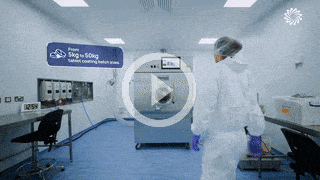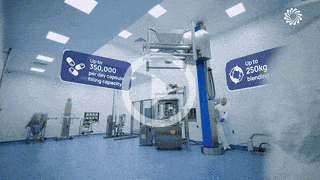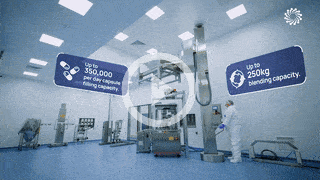
An award-winning facility
built to meet your patient targets.
Our award-winning 50,000 sqft development and manufacturing facility, Trent Gateway, enables our teams to be flexible and nimble so that we can problem-solve, meet your timescales, and deliver solutions that meet patient targets.

Formulation Development
Our team has extensive experience in selecting the correct oral, nasal and pulmonary dosage forms to ensure success in getting to the clinic.
- Formulation and analytical development
- Toxicology supplies
- ASAP stability to support clinical prototype selection
Phase 1 – Phase 2 Clinical Supply
We can help you accelerate to Phase 1 clinical trials through formulation optimisation and identify the next steps to transition into Phase 2 clinical trials following Phase 1 data.
- Clinical manufacturing and QC testing
- Qualified Person Release
- Clinical stability
Process Scale-Up for Phase 3
We understand the complexities of scale up and technical transfer. Covering all aspects from equipment consideration through to manufacturing handover and QC.
- Process optimisation robustness (Qbd, DoE)
- Method validation
- Cleaning validation
Registration Batches
We support your product’s journey to market with comprehensive registration activities, including preparing and submitting NDA/BLA regulatory filings to streamline your approval process.
- NDA/BLA submission
- Batch manufacture and stability
- Analytical support (dissolution development / risk assessments)
Analytical Development & Validation
Our dedicated QC and analytical teams provide phase appropriate validation of your analytical methods and performance of QC and release testing including:
- Assay, Content uniformity and related substances by HPLC
- Performance testing including Dissolution and Disintegration
- IC Stability testing
Regulatory Affairs Support
Dedicated regulatory resources to protect your confidentiality that spans the following phases of your products development pathway.
- Clinical trials
- Regulatory strategy
- Gap analysis
- Classifications
- Marketing applications
Working with Upperton has been exceptional. Their project management approach is marked by outstanding organisation, proactive communication, and a deep commitment to meeting project milestones.
Client Testimonial
At Upperton, we deliver science-led solutions from pre-clinical to market, offering expertise in oral, nasal, and pulmonary dosage forms. Backed by our award-winning Trent Gateway facility and a team of subject-matter experts, we ensure collaboration, agility, and success at every stage of your drug development journey.
Book a discovery call today and see how we can make a difference.
Speak with our Experts
Get in touch to start a discovery call with our team and accelerate your drug program journey with Upperton.






