- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Choosing Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Our History.
Discover the milestones that have shaped
the company we have become today.
1999
Established.
Upperton Limited was formally established on August 31st, 1999.
Dr. Richard Johnson, co-founder of the drug delivery company Andaris, initiated the company’s inception. Initially operating as a consultant under the Upperton Limited name, Dr. Johnson provided specialised technical consultancy services centred on spray drying and cleanroom technology, catering to clients across the UK and Europe.


2001
Research Resurgence.
In 2001, Dr. Johnson’s focus shifted towards the development of innovative imaging products, particularly in the realm of spray drying diagnostic imaging as he returned to laboratory work. This endeavor prompted the establishment of a laboratory at Nottingham University within the Department of Medical Physics.
Continued growth led to the expansion of operations in 2002, with Upperton securing larger laboratory premises. This period marked a concerted effort towards proprietary product development utilising advanced spray drying technology.
2006
Strategic Shift.
A strategic transition occurred in 2006, as Upperton shifted its focus from proprietary research to specialised contract services.
In a significant move reflecting our evolution within the pharmaceutical landscape, the company rebranded in 2010 as Upperton Pharma Solutions.


2014
Collaborative Ventures.
Upperton’s participation in the EU Horizon 2020 Grant consortium in 2014 marked a pivotal milestone.
Collaborative efforts aimed at developing nasal and oral HIV vaccines utilising spray drying technology underscored the company’s commitment to innovation and collaboration.
2018
GMP Facilities.
The establishment of GMP facilities at the Albert Einstein Centre, Nottingham, in 2018, and a successful MHRA accreditation, marked a significant milestone in Upperton’s journey, solidifying its status as a GMP-certified entity committed to upholding the highest standards of quality and compliance.

2022
Investment and Growth.
In 2022, Upperton Pharma Solutions received significant development capital investment from Inflexion, signalling a new era of growth and expansion for the company.
Over the coming year, the headcount increased from 40 to 90 employees with an influx of pharmaceutical talent, alongside a significant expansion in its scale and capabilities through investment in state-of-the-art equipment.


2023
New Facility Build.
In January 2023 the build of a new 50,000 sqft development and manufacturing facility in Beeston, Nottingham started.
The facility benefited from a £20 million investment in commercial scale equipment, offering powder blending up to 250kg per batch, capsule filling up to 40,000 capsules/hour, dry granulation processing up to 100kg/hour, and tablet pressing up to 120,000 tablets/hour.
2023
King's Award Winner.
In August 2023, Upperton was honored with the King’s Award for Enterprise in International Trade, marking a notable achievement.
This recognition reflects Upperton’s remarkable growth since 2020 and its dedication to enhancing global partnerships within the pharmaceutical sector.


2023
Facility Build Complete Within 10 Months.
In October 2023, the Trent Gateway facility construction was successfully finished in an unprecedented timeframe of just 10 months.
This achievement marks a significant milestone for Upperton, providing a substantial increase in manufacturing footprint from 1,300 sq.ft. to 13,000 sq.ft., along with a notable expansion of analytical laboratory space from 880 sq.ft. to 8,500 sq.ft.
The new site supports early formulation development, clinical trial supplies ranging from Phase 1 to Phase 3, and niche-scale commercial manufacturing.
2024
Milestone Achievement.
The culmination of Upperton’s journey thus far was celebrated with the official opening of Trent Gateway as the ‘The Richard Johnson Building’ in April 2024, serving as a testament to the company’s resilience, innovation, and unwavering commitment to excellence.

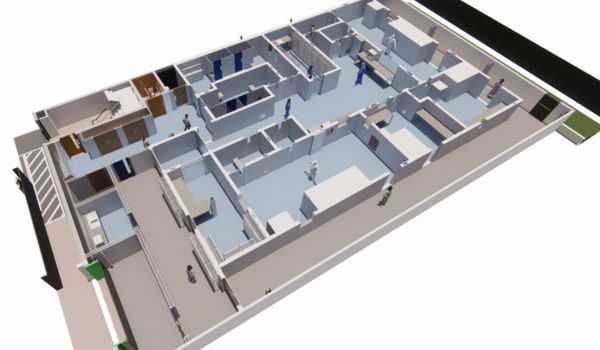
2024
Sterile Facility Expansion.
In 2024, Upperton announced further expansion at its Trent Gateway site to include sterile capabilities with a purpose-built facility designed specifically in line with the revised EU GMP Annex-1 regulations.
The £5million investment into an additional 7,000sqft sterile manufacturing facility follows a period of significant growth in demand for the formulation development and manufacture of sterile and terminally sterilised products.
2024
Sunday Times 100
Upperton was recognised as one of the Sunday Times’ 100 fastest-growing companies in the UK for 2024.






