The Benefits of Powder Characterisation
When developing products containing spray dried powders it is vital that the physical properties of the powders produced are determined.
The physical properties of spray dried powders provides important information that can assist during the pre-formulation screening and early process development phases since these properties will have a direct impact on the attributes of the final product.
Testing typically falls into two categories:
- Tests that analyse amorphous/crystalline content and the propensity for moisture uptake; characteristics that will identify potential handling/product stability issues.
- Tests on particle size, morphology and powder flow properties that will impact on handling properties and aerodynamic performance of the spray dried powders.
The physical properties of the spray dried powders will be primarily influenced by a number of factors such as the properties of the API, the solvents/excipients present in the feed solution (and subsequently the spray dried powders produced) and the process parameters used in the spray drying process.
Many of these processing parameters can be adjusted/optimised to obtain the desired physical features hence there is a need to undertake tests at an early stage in the development process.
Ultimately the goal is to create spray dried powders that have the desired physical properties necessary to ensure a successful outcome for the final dosage form (eg tablet, capsule or nasal/pulmonary device) whilst maintaining a good stability profile.

Testing of Spray Dried Powders at Upperton
At Upperton we analyse a number of physical tests on spray dried powders from the earliest stages in the development process. These usually fall into two basic categories ie (i) tests that investigate the crystal/amorphous content of spray dried powders and their interaction with solvents and (ii) tests that evaluate the size/morphology of the spray dried powders produced.
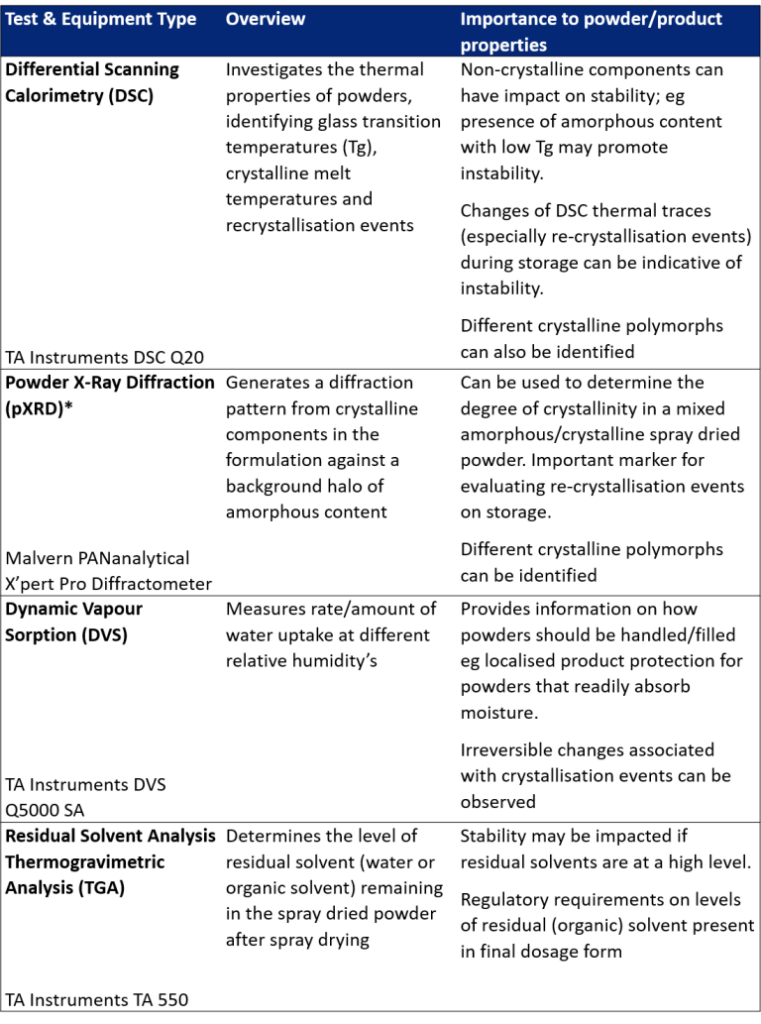
Tests that investigate the amorphous/crystallisation content of spray dried powders and their interaction with solvents
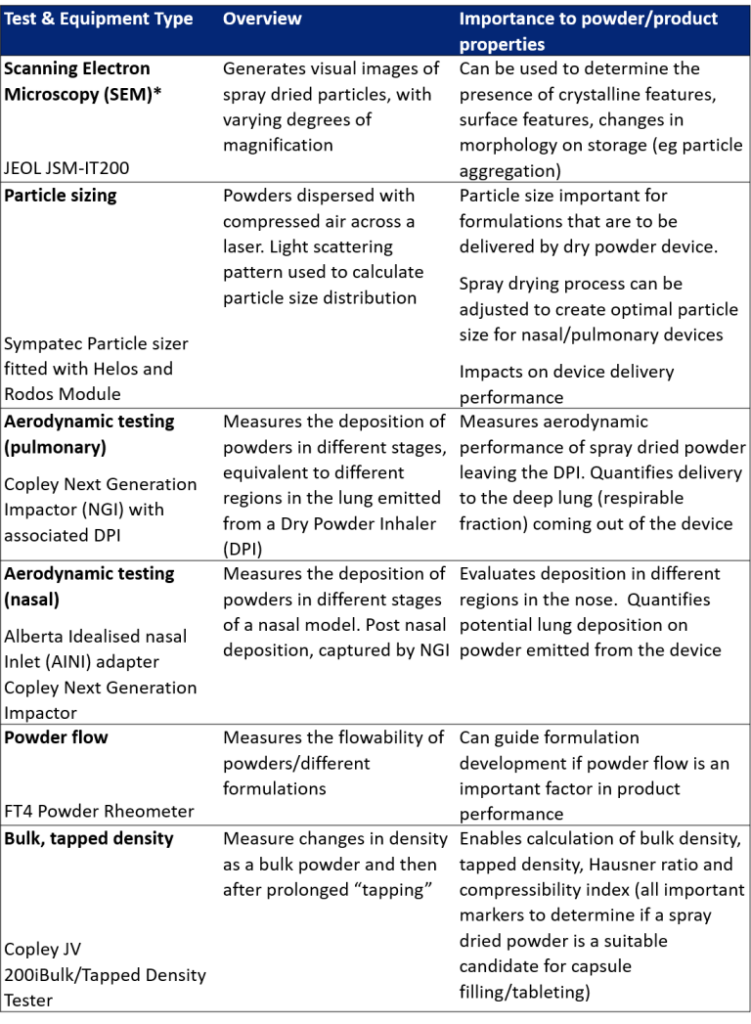
Tests that evaluate particle size, morphology and aerodynamic performance of spray dried powders
Conclusion
Testing the physical properties of spray dried powders provides important information on the suitability of a spray dried powder for further dosage form development.
A wide range of assays are available, each providing important insight into the stability and handling properties of spray dried powders, hence it is important that physical testing of these powders is undertaken from the early stages of the development process.
Early testing is particularly important since indications of instability of spray dried powders and inferior handling or aerodynamic properties can be highlighted. These challenges can quite often be overcome by changes in solvents, excipient selection and by adjusting spray drying processing conditions.
Get in touch.
If you’re looking to work with a CDMO that can support your product from preclinical development to market and beyond, then we’re here to help.





