- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Nasal Dosage Forms.
Nasal Delivery of Small Molecules and Biologics.
Expertise
Our Leadership team has extensive collective experience in steering products from pre-clinical to commercial manufacture.
Science-led
Our approach is scientific, collaborative and open, ensuring an open dialogue and transparency that fits your project.
Award-winning
We are a leading CDMO recognised for numerous awards including a King's Award For Enterprise.
Nimble
We adapt to your project needs, and pledge to never relegating it to the bottom of the list.
The ability to self-administer small molecules, biologics and vaccines is highly attractive yet the knowledge and expertise necessary to rapidly develop products for this alternative delivery route remains limited in the CDMO space. To meet this growing demand, Upperton has developed UpperNose™, a unique nasal product development platform. Our aim is to make it easier and faster for small and mid-sized innovators to access the knowledge and capabilities necessary for the successful development of nasal dosage forms.
The UpperNose™ platform can be applied to liquid and dry powder formulations and utilises the knowledge and expertise of the highly experienced Upperton scientific team to develop your molecule in an efficient and timely manner.
Working closely with our clients, we quickly identify the most time/cost effective development programme to facilitate early, smooth passage of our customer’s molecule from early-stage development into clinical trials. Typically we will take our customers through a series of well-established development steps although in reality, some of these can be combined or omitted depending on the dosage form required.
Applications
- Local delivery to the nasal membranes (e.g. for vaccines, antivirals, prophylactics)
- Systemic delivery by absorption into the bloodstream (e.g. for fast action whilst avoiding first pass metabolism)
- Direct delivery into the brain (transcending the blood brain barrier for treatment of neurodegenerative and psychological disorders)
The UpperNose™ technology platform provides our customers with a progressive, data-driven approach to accelerate their drug into nasal delivery testing with the optimal dosage form design and the best chances of success in the clinic and beyond.
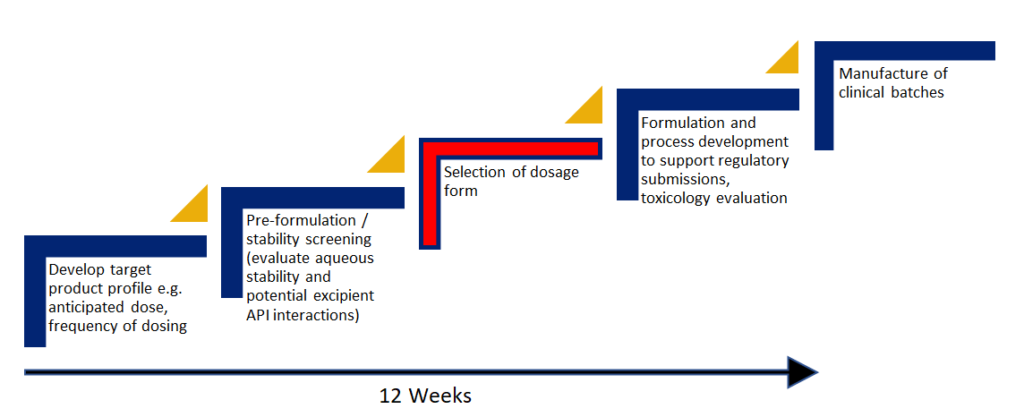
The UpperNose™ Development Pathway
Develop target product profile e.g anticipated dose, frequency of dosing
Pre-formulation / stability screening
Selection of dosage form
Formulation and process development to support regulatory submissions, toxicology evaluation
Manufacture of clinical batches
Benefits of UpperNose™
- Generating scientific and regulatory data to get you into the clinic with a high degree of confidence. Data to make informed decisions and support regulatory submissions.
- Driven by experts and led by science. Our highly experienced team will work with you to overcome the unique needs of your molecule.
- Working with customer’s own or commercially available nasal delivery devices. Ensuring the successful development of your drug either at the development or clinical trial phase.
- Laying the foundation for commercial success. We will help you establish a clear path that could take you all the way to commercial success. Reach your milestones faster without compromising quality.
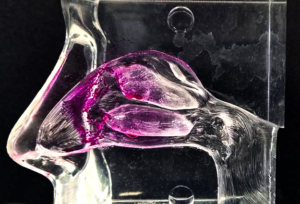

Applications
- Local delivery to the nasal membranes (e.g. for vaccines, antivirals, prophylactics)
- Systemic delivery by absorption into the bloodstream (e.g. for fast action whilst avoiding first pass metabolism)
- Direct delivery into the brain (transcending the blood brain barrier for treatment of neurodegenerative and psychological disorders)
The UpperNose™ Development Pathway
Benefits of UpperNose™
- Generating scientific and regulatory data to get you into the clinic with a high degree of confidence. Data to make informed decisions and support regulatory submissions.
- Driven by experts and led by science. Our highly experienced team will work with you to overcome the unique needs of your molecule.
- Working with customer’s own or commercially available nasal delivery devices. Ensuring the successful development of your drug either at the development or clinical trial phase.
- Laying the foundation for commercial success. We will help you establish a clear path that could take you all the way to commercial success. Reach your milestones faster without compromising quality.
The UpperNose™ technology platform provides our customers with a progressive, data-driven approach to accelerate their drug into nasal delivery testing with the optimal dosage form design and the best chances of success in the clinic and beyond.
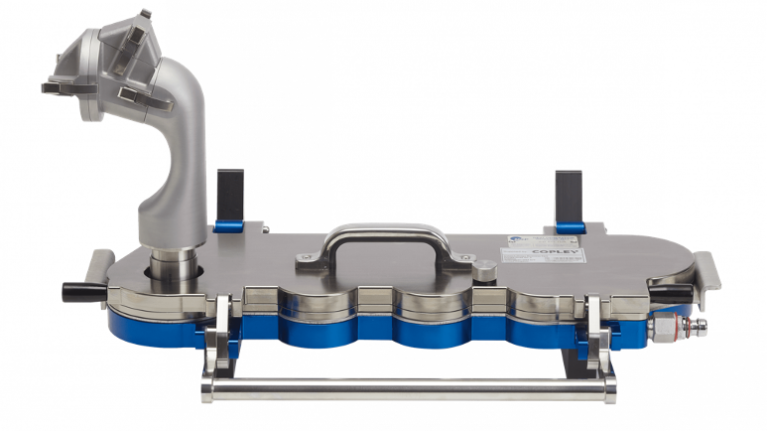
Copley AINI device attached to the Next Generation Impactor (NGI)
We're here to help
If you’re looking to work with a CDMO that can support your product from preclinical development to market and beyond, then we’re here to help.
Speak to our team to discuss your requirements.
Access the knowledge and capabilities necessary for the successful development of nasal dosage forms.
- The vestibule: small surface area and squamous epithelial cell surface make it unsuited to extensive drug absorption.
- The nasopharynx-associated lymphatic tissue (NALT), a part of the mucosal immune system, acts to prevent infection (target for for intranasal vaccination via T-cell immunity,
- The turbinates are the largest (approximately 130 cm2) and most vascularized part of the nasal cavity and a prime site for systemic drug absorption after nasal administration.
- The olfactory region comprises a small fraction (≤10 %) of the total area of the nasal cavity, 2–12.5 cm2 . Contains olfactory sensory neurons (OSNs), the only site where the CNS is in direct contact with the outer surface of the body, the nasal mucosal membrane in this case, and thus opens the possibility of direct-to-brain delivery of drugs (via the paracellular space between olfactory axons).
Nasal Drug Delivery
Experience robust growth in the nasal drug delivery market, anticipated to surge throughout this decade.
The Global Nasal Drug Delivery Technology market, boasting a valuation of US$50.9 billion in 2022, is primed for substantial expansion. Projections indicate a remarkable ascent, with the market forecasted to soar to US$75.7 billion by 2030, reflecting a notable compound annual growth rate (CAGR) of 5.8% from 2022 to 2030*.
This trajectory underscores the growing significance and potential of nasal drug delivery mechanisms in meeting healthcare demands and driving innovation within the pharmaceutical sector.

Generating scientific and regulatory data to get you into the clinic with a high degree of confidence.
Data to make informed decisions and support regulatory submissions.
Driven by experts and led by science.
Our highly experienced team will work with you to overcome the unique needs of your molecule.
Working with customer’s own or commercially available nasal delivery devices.
Ensuring the successful development of your drug either at the development or clinical trial phase.
Laying the foundation for commercial success.
We will help you establish a clear path that could take you all the way to commercial success. Reach your milestones faster without compromising quality.
Target product profile
Development of the target product profile including anticipated dosage form and frequency of dosing. Activities include API solubility and stability screening.
Formulation Development
ASAP stability, In vitro diffusion, cell membrane studies and AINI deposition
Clinical Development
Clinical package IMPD/IMP, Method Qualification, ICH stability, Tech batches