- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Choosing Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Expertise.
The technical expertise and scientific staff
to make your development a reality
Expertise
Our Leadership team has extensive collective experience in steering products from pre-clinical to commercial manufacture.
Science-led
Our approach is scientific, collaborative and open, ensuring an open dialogue and transparency that fits your project.
Award-winning
We are a leading CDMO recognised for numerous awards including a King's Award For Enterprise.
Nimble
We adapt to your project needs, and pledge to never relegating it to the bottom of the list.
Our Formulation Work is Driven by our Expertise
In our MHRA approved facility, we offer manufacturing and packaging of Investigational Medicinal Product (IMP) in dosage forms including oral solid dosage forms, bulk dry powders, dry powder inhalers, nasal suspensions and solutions; for both biological molecules and traditional small molecules. Placebo development and manufacture are also offered.
Spray Drying
We have been successfully developing spray dried formulations for our customers for more than twenty years. Our pharmaceutical expertise and experience spans a vast range of therapeutics from small molecule API’s to even the most sensitive and complex biologicals.


Oral Dosage Form Development
We have extensive experience and know-how in the development of oral dosage forms containing spray dried powders. Tablets and capsules are the most common dry powder dosage form. However, other dosage forms such as sachets (bulk powders), vials (for reconstitution) have also be developed by the Upperton team.
Pulmonary Delivery
We are seeing increased interest in delivering APIs and biologics into the lung as either a liquid or dry powder dosage form. Use our knowledge and expertise to develop dosage forms with optimal aerodynamic properties tailored to the delivery device.

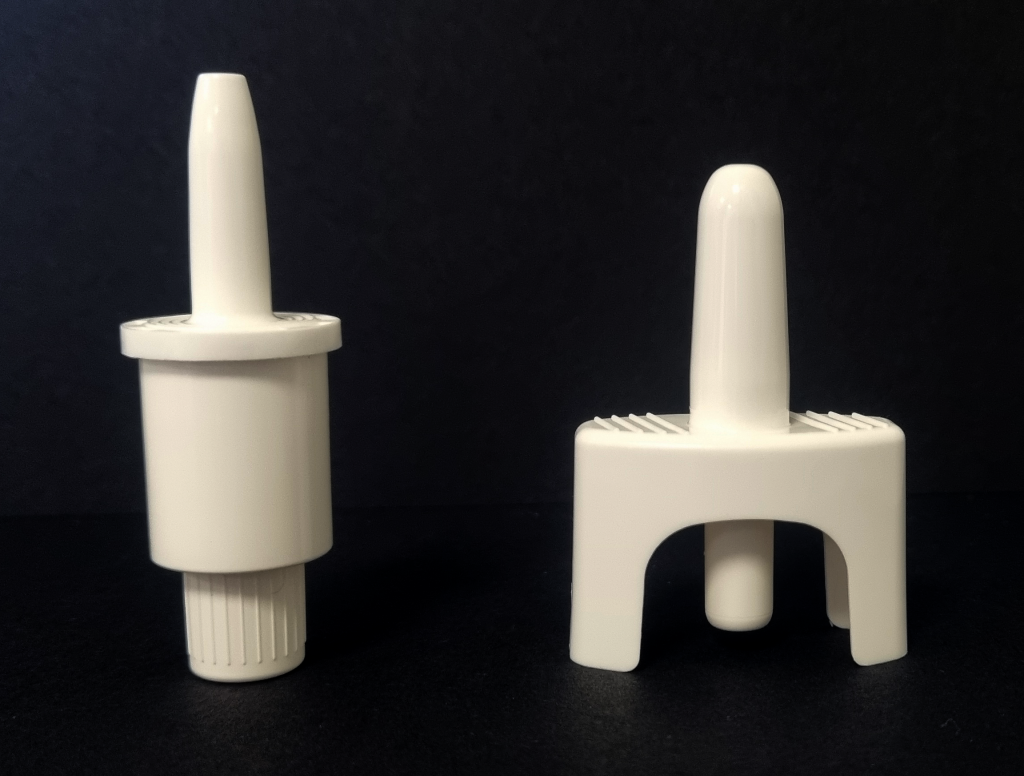
Nasal Dosage Form Development
The ability to self-administer small molecules, biologics and vaccines via the nasal route is highly attractive yet the knowledge and expertise necessary to rapidly develop products for this delivery route remains limited in the CDMO space. Talk to Upperton about our UpperNose™ delivery platform focussed on the successful development of nasal dosage forms.
Our Formulation Work is Driven by our Expertise
Upperton have the technical pharmaceutical expertise to help make your development a reality. With highly experienced scientific staff which utilise spray drying and analytical techniques to develop dry dosage forms, such as sachets of dry powders, tablets, capsules, nasal and pulmonary delivery.
Our ability to recruit and retain good scientists; along with our knowledge around our core technology, spray drying has enabled us to build up trust with our customers.
See below our key areas of expertise in spray drying, oral solid dosage forms, nasal and pulmonary delivery. Upperton also offer virtual seminars to companies who wish to understand more on these topics.

Spray Drying
We have been successfully developing spray dried formulations for our customers for more than twenty years. Our pharmaceutical expertise and experience spans a vast range of therapeutics from small molecule API’s to even the most sensitive and complex biologicals.
Oral Dosage Forms
We have extensive experience and know-how in the development of oral dosage forms containing spray dried powders. Tablets and capsules are the most common dry powder dosage form. However, other dosage forms such as sachets (bulk powders), vials (for reconstitution) have also be developed by the Upperton team.


Pulmonary Delivery
We are seeing increased interest in delivering APIs and biologics into the lung as either a liquid or dry powder dosage form. Use our knowledge and expertise to develop dosage forms with optimal aerodynamic properties tailored to the delivery device.
Nasal Delivery
The ability to self-administer small molecules, biologics and vaccines via the nasal route is highly attractive yet the knowledge and expertise necessary to rapidly develop products for this delivery route remains limited in the CDMO space. Talk to Upperton about our UpperNose™ delivery platform focussed on the successful development of nasal dosage forms.

What is CMC in Drug Development?
CMC refers to the detailed documentation and processes required to manufacture a drug safely and consistently.






