- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
UpperSolv™.
Identifying a Formulation Strategy to Maximise Success in the Clinic
Expertise
Our Leadership team has extensive collective experience in steering products from pre-clinical to commercial manufacture.
Science-led
Our approach is scientific, collaborative and open, ensuring an open dialogue and transparency that fits your project.
Award-winning
We are a leading CDMO recognised for numerous awards including a King's Award For Enterprise.
Nimble
We adapt to your project needs, and pledge to never relegating it to the bottom of the list.
Many poorly soluble drugs undergoing clinical trials fail due to insufficient bioavailability, as a result it is therefore apparent that low bioavailability risk mitigation must occur during the earlier stages of drug formulation development in order to reduce time to clinic, maximise development budgets and halt the premature dropping of promising molecules.
Low bioavailability is a frequent issue when formulating orally administered dosage forms. With the most commonly discussed cause being poorly soluble drugs.
Developing a strong understanding early in drug development is key to preventing common reasons for failure and producing enabled formulations to maximise outcomes. Our early formulation platform, UpperSolv™ outlines a parallel integrated formulation and biopharmaceutical screening protocol that can rapidly compare a range of enabling formulation strategies that can be translated into clinical processes and products.
UpperSolv™ is a signposted pathway to rapid, tailored screening of small quantities of API, the manufacture of prototype formulations using a range of scalable enabling technologies and comparison of the biopharmaceutical performance via a PK assessment.
The UpperSolv™ Process
The poor water solubility of an API can be characterised by either of the following mechanisms:
A. Dissolution rate limited, where the surface area to particle volume is too high.
B. Solubility rate limited, where the API is in a stable state (e.g. in a crystalline form).
When poor water solubility is limited by its dissolution rate, simple particle size reduction resulting in an increased surface area is likely to improve its solubility and in many this is sufficient to achieved the required exposure. This particle size reduction is readily accomplished by micronisation which is a well established and easy to access manufacturing operation.
For APIs limited by their solubility rate, then a more comprehensive process of identifying the most appropriate formulation strategy will be necessary.
UpperSolv™ can signpost the way with the following protocol:
1. Solubility screen where the API is dissolved with a range of solvents, co-solvents and solubilising excipients.
2. Applying enabling technologies to produce formulated intermediates and /or prototype product that have improved solubility. These include:
- Lipid based formulations (e.g. the API solubilised in a lipid based carrier is filled into capsules)
- Amorphous formulations created by co-milling
- Amorphous Solid Dispersions created by spray drying
3. Comparative in vitro characterisation and testing of the prototype enabled formulation followed by stability testing.
4. Comparative pharmacokinetic assessment (PK study) in small animal models.
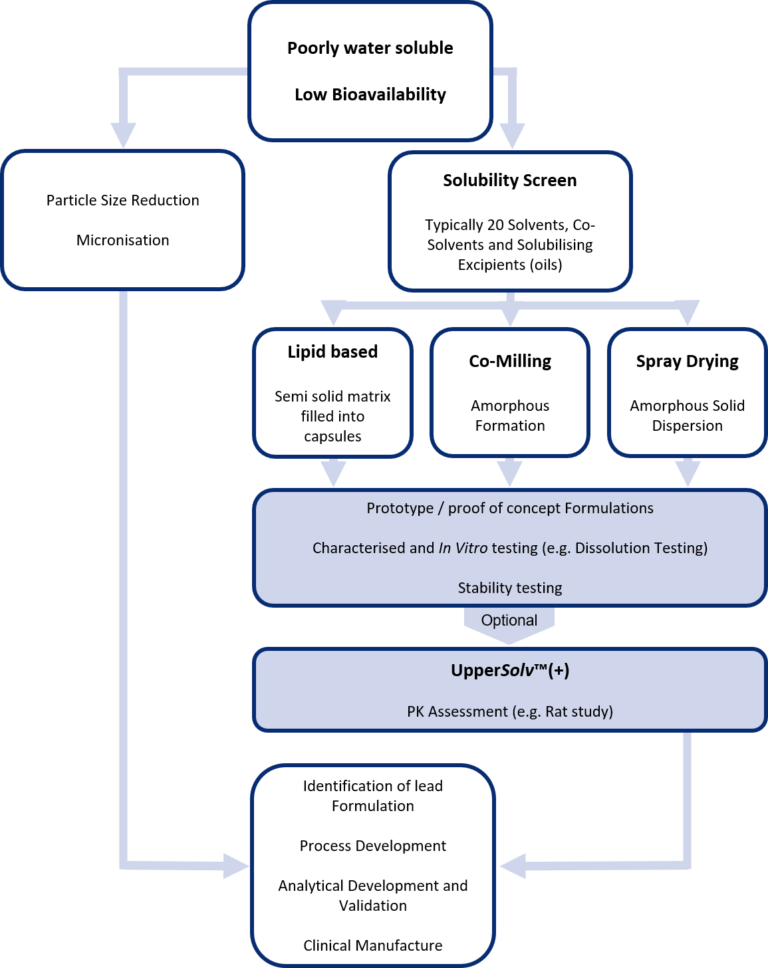
By applying the UpperSolv ™ protocol, an array of informative data will be generated to support a science and data driven informed decision to made on which formulation strategy is most likely to succeed in the clinic and support development into a registered product for patients.
The UpperSolv™ Protocol
- Requires minimal API – typical 5 grams will support the testing of up to 6 formulations
- Rapid turnaround – the entire study including generation of PK data will typically be complete in 8 weeks
- Adaptive, tailored approach depending on the molecule and client objectives
- Combined formulation and biopharmaceutical approach to selecting the most appropriate formulation strategy
UpperSolv ™ is the signpost that will direct the selection of the most appropriate formulation strategy to maximise the opportunity for success in the clinic and ultimately to the development of a product available to patients.
The advantages of UpperSolv™
- Rapid comparative PK assessment to support formulation strategy selection.
- Rapid evaluation of a range of enabling technologies achieved with minimal drug.
By applying the UpperSolv ™ protocol, an array of informative data will be generated to support a science and data driven informed decision to made on which formulation strategy is most likely to succeed in the clinic and support development into a registered product for patients.
The UpperSolv™ Process
The poor water solubility of an API can be characterised by either of the following mechanisms:
A. Dissolution rate limited, where the surface area to particle volume is too high.
B. Solubility rate limited, where the API is in a stable state (e.g. in a crystalline form).
When poor water solubility is limited by its dissolution rate, simple particle size reduction resulting in an increased surface area is likely to improve its solubility and in many this is sufficient to achieved the required exposure. This particle size reduction is readily accomplished by micronisation which is a well established and easy to access manufacturing operation.
For APIs limited by their solubility rate, then a more comprehensive process of identifying the most appropriate formulation strategy will be necessary.
UpperSolv™ can signpost the way with the following protocol:
1. Solubility screen where the API is dissolved with a range of solvents, co-solvents and solubilising excipients.
2. Applying enabling technologies to produce formulated intermediates and /or prototype product that have improved solubility. These include:
- Lipid based formulations (e.g. the API solubilised in a lipid based carrier is filled into capsules)
- Amorphous formulations created by co-milling
- Amorphous Solid Dispersions created by spray drying
3. Comparative in vitro characterisation and testing of the prototype enabled formulation followed by stability testing.
4. Comparative pharmacokinetic assessment (PK study) in small animal models.

By applying the UpperSolv ™ protocol, an array of informative data will be generated to support a science and data driven informed decision to made on which formulation strategy is most likely to succeed in the clinic and support development into a registered product for patients.
The UpperSolv™ Protocol
- Requires minimal API – typical 5 grams will support the testing of up to 6 formulations
- Rapid turnaround – the entire study including generation of PK data will typically be complete in 8 weeks
- Adaptive, tailored approach depending on the molecule and client objectives
- Combined formulation and biopharmaceutical approach to selecting the most appropriate formulation strategy
UpperSolv ™ is the signpost that will direct the selection of the most appropriate formulation strategy to maximise the opportunity for success in the clinic and ultimately to the development of a product available to patients.
The advantages of UpperSolv™
- Rapid comparative PK assessment to support formulation strategy selection.
- Rapid evaluation of a range of enabling technologies achieved with minimal drug.
Get in touch
If you’re looking to work with a CDMO that is flexible and nimble, then we’re here to help.