

The Challenge of Stabilising Injectable Biologics
Therapeutic proteins, such as monoclonal antibodies (mAbs), are increasingly used in injectable formulations to treat a wide range of conditions. However, their complex structures make them particularly vulnerable to denaturation, aggregation, and chemical degradation. These are issues that can compromise safety and drug efficacy.
Formulating injectable buffers that stabilise these biologics is critical but challenging. Injectable buffers work to stabilise proteins by enhancing non-covalent interactions. Thus, protecting against environmental factors, and minimising chemical instabilities such as oxidation, reduction and deamidation. Traditional methods for buffer screening (e.g., buffer exchange and circular dichroism) are often low-throughput, time-consuming, and resource-intensive.
At Upperton Pharma Solutions, we see thermal shift fluorimetry (thermofluor) techniques as having the potential to offer a rapid, low-volume, and low-cost alternative to quickly assess injectable buffers for protein stability optimisation.
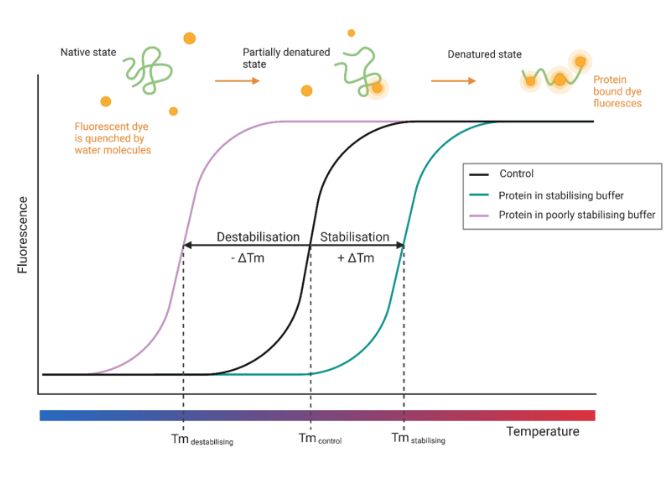
A Thermofluor-Based Screening Assay
To support high-throughput formulation development, we investigated the suitability of a 96-well thermofluor assay to aid in the formulation of injectable protein biologics. The method uses SYPRO™ Orange dye, which binds to hydrophobic regions of denaturing proteins as temperature increases, and produces a fluorescent signal that reveals melting temperature (Tm), a key indicator of protein stability. This study then aimed to validate an example optimised buffer via Dynamic Light Scattering (DLS) to assess for aggregation prevention of a monoclonal antibody.
Methodology
To evaluate the stability of a model protein under a wide range of conditions, a 96-well thermofluor screening array was constructed using protein-stabilising additives and buffers approved for SC, IM or IV injections within the FDA’s Inactive Ingredient Database (2025). A humanised monoclonal antibody (mAb1) at 25 µM was combined with 50X SYPRO™ Orange dye in a 4:1 v/v ratio and screened across these additives using a thermal shift assay (TSA). Each 12.5 µL of protein-dye mix was combined with an equal volume of 2X additive or buffer solution in wells (n=3, N=2) and subjected to a controlled temperature ramp on a Bio-Rad thermal cycler, with real-time fluorescence detection to determine protein melt temperatures (Tm) via Boltzmann curve fitting in wTSA-CRAFT.
The most stabilising additives and buffer agents (based on positive ΔTm values) were then combined to formulate an ‘optimised buffer’ including selected surfactants, amino acids, sugars, and co-solvents. mAb1 was subsequently prepared in this buffer at 5 mg/mL and assessed for thermal stability via dynamic light scattering (DLS) after incubation at 70°C for two hours. Stability profiles were analysed using ZS Xplorer and GraphPad Prism, with statistical significance determined via one-way ANOVA.
Key Findings and Results
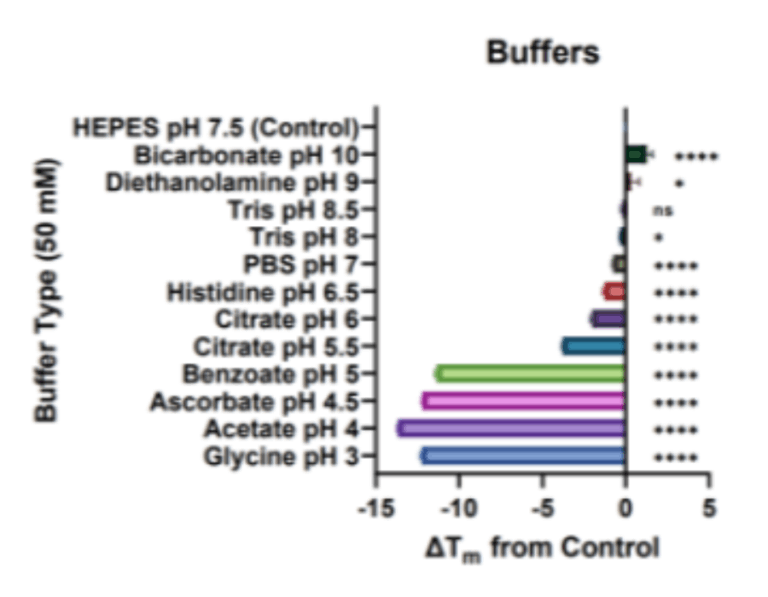
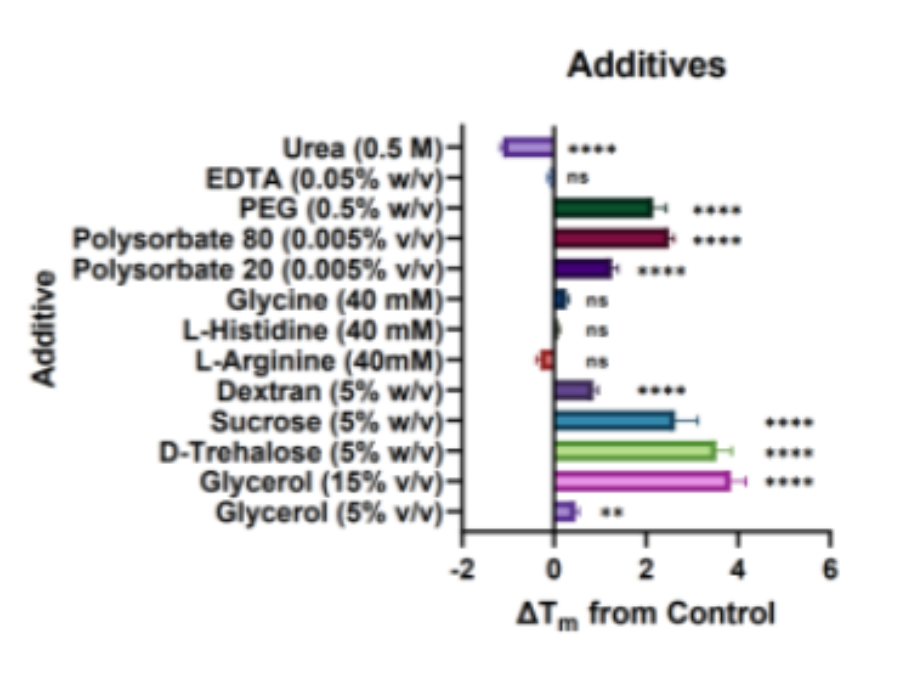
Figure 2: ΔTm observations for mAb1 protein in a range of (a) physiologically compatible 50mM buffers and (b) protein stabilising additives and a urea negative control in a 50mM HEPES buffer with pH 7.5. Horizontal bars represent the change in protein melt temperature ΔTm (mean ± SD, N=2, n=3) against the control buffer of 50mM HEPES with pH 7.5. Positive values indicate a stabilising effect. One-way ANOVA was performed where ns signifies not significant; * p<0.05, **p≤0.01; ****p≤0.0001.
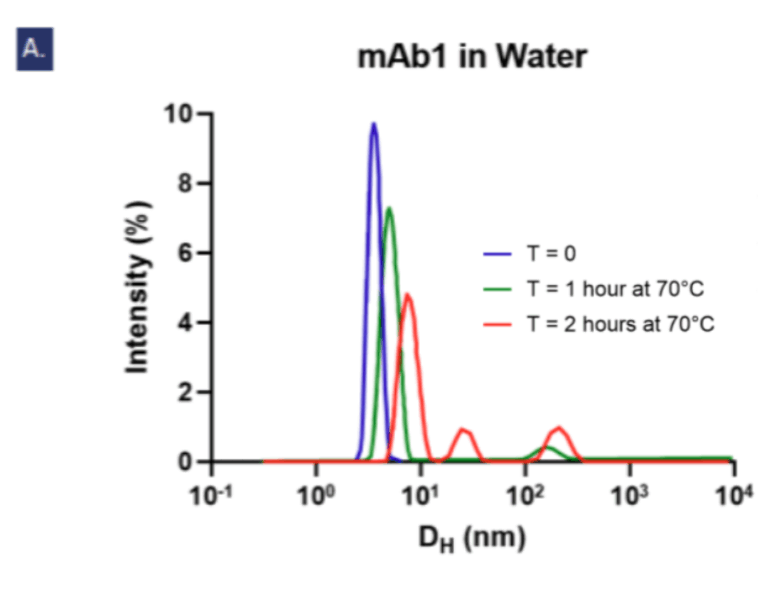
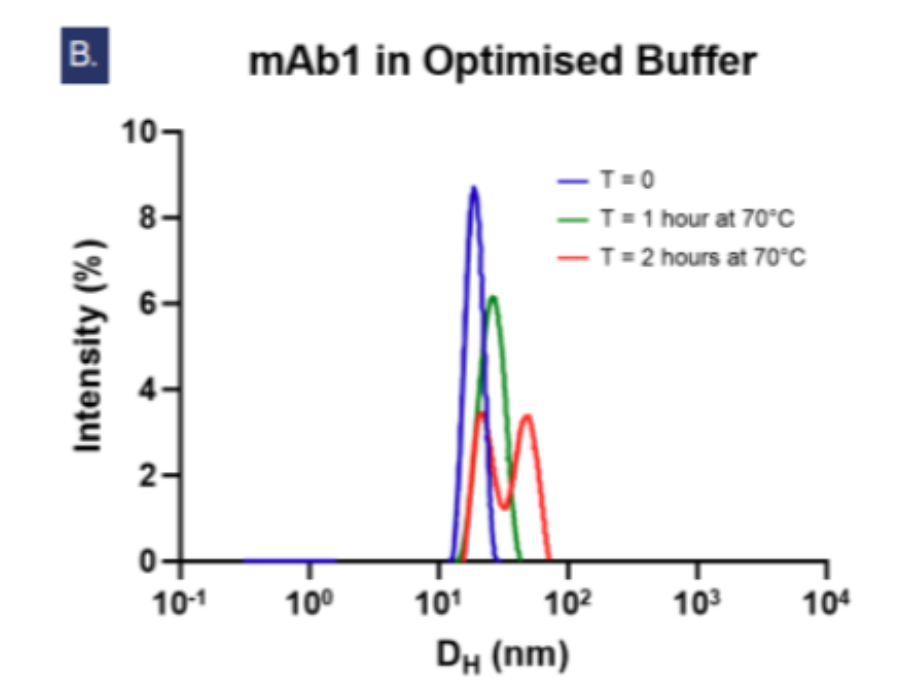
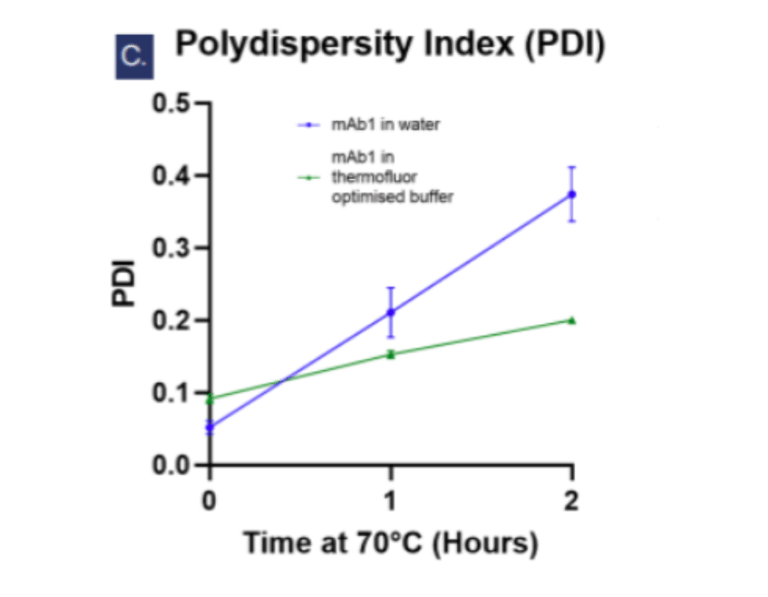
Figure 3: Dynamic light scattering intensity graphs showing Figue 3: Dynamic lightly scattering intensity graphs showing the particle hydrodynamic diameter (DH) measurements of 5 mg/ml mAb1 solutions over 2 hours at 70°C (n=3, N=2). At T=0, the average DH of mAb1 in water (a) is 3.466 nm which is smaller than in the optimised buffer (b) at 17.982 nm. In water, an aggregate peak >100 nm appears after 1 hour and grows alongside a second peak at ~27nm by the second hour, but in the optimised buffer (b) no aggregate peaks >100 nm are observed. Fig. c shows the increases of polydispersity index over time signifying aggregation is accelerated in water compared to in the optimised buffer.
Optimising Protein Formulations with Thermofluor Screening
Thermofluor screening proved to be a practical and repeatable method for guiding rational buffer design, identifying conditions that enhanced the thermal stability of a model monoclonal antibody (mAb1). The optimised formulation comprising 50 mM HEPES (pH 7.5), 0.005% Polysorbate 80, 40 mM Glycine, 5% D-trehalose, and 15% Glycerol was selected for the stability profiles of the individual components and physiological relevance of the pH.
Compared to ultrapure water, the optimised buffer significantly reduced aggregation (PDI: 0.200 ± 0.002 vs. 0.374 ± 0.037, p < 0.001) and limited the formation of large or heterogeneous aggregate species after thermal stress. The slower rate of aggregation in this buffer further highlighted the protective effect.
The versatility of a 96-well thermofluor platform allows for the high throughput testing of buffers or entire formulations and could be readily paired with complementary techniques like DLS or circular dichroism for accelerated formulation development and resource optimisation.
Areas For Further Research
This thermofluor workflow could potentially be extended beyond the model mAb protein to other protein-based therapeutics, enabling fast formulation optimisation using readily available, regulatory-approved excipients.
Further research should focus on expanding this platform with additional analytical techniques and experimental strategies, including:
- Design of Experiments (DoE) studies to detect synergistic interactions between buffer components.
- Zeta potential measurements to evaluate the impact of ionic strength and electrostatic stability on protein aggregation behaviour.
Collectively, these approaches will significantly advance the development of safe, efficacious, and stable injectable protein-based biologics. By reducing the risk of aggregation and degradation, they contribute to improved patient outcomes and help ensure biologic therapies reach the clinic in their most effective form.
Get in touch.
If you’re looking to work with a CDMO that can support your product from preclinical development to market and beyond, then we’re here to help.
Speak to our team to discuss your requirements.
If you’re looking to work with a CDMO that can support your product from preclinical development to market and beyond, then we’re here to help.