
Richard Johnson
Founder & Chief Scientific Officer
Nasal Drug delivery was once considered suitable only for a limited number of niche products. These were primarily focussed on treating conditions related to the nasal epithelium, such as allergic rhinitis. However, nasal drug delivery is now emerging as a promising route for the rapid delivery of certain classes of molecules via the nose directly into the systemic circulation. This is particularly useful where patients require rapid onset (such as with pain relief, hypoglycaemia and the onset of seizures) with the added advantage of self-administration rather than injection.
Alongside these systemic delivery approaches there is growing interest in targeting the brain via the nasal route. In doing so, avoiding the BBB (blood brain barrier) thanks to recent research into the unique properties of the olfactory nasal epithelium.
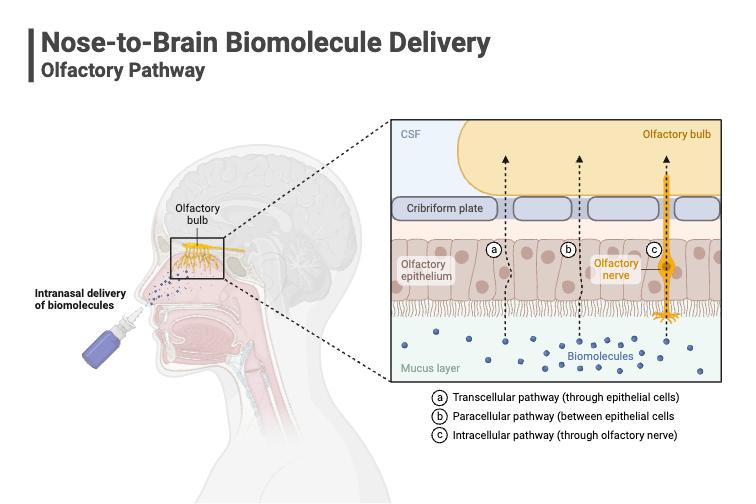
This specialised tissue forms a direct anatomical and physiological link between the external environment and the central nervous system (CNS), offering a novel pathway for therapeutic intervention. As a result, nasal delivery is becoming a strategic focus in the development of delivery options for drugs that need to target the brain/CNS drug
Despite its promise, nasal drug delivery presents a number of complex challenges. Anatomically, the nasal cavity is difficult to navigate and delivering drugs to brain involves using the connectivity of the olfactory and trigeminal nerve, which is particularly demanding. Physiologically, the nasal environment is designed to protect the body from foreign substances, which means it actively works against drug absorption. Mucociliary clearance, for example, rapidly removes particles from the nasal cavity, limiting the time available for drug uptake.
From a formulation perspective, developers must also contend with constraints around drug loading capacity, delivery volume, and efficiency of deposition. Achieving consistent and targeted delivery to the upper nasal regions where nose-to-brain absorption occurs requires precision-engineered devices and carefully optimised formulations.
"One route into the brain is to avoid the blood brain barrier altogether through the delivery of drugs directly into the brain following the administration of a drug into the nose."
Dr Richard Johnson, Chief Scientific Officer
A Market on the Move
According to Coherent Market Insights, the global nasal drug delivery technology market was valued at US$50.9 billion in 2022 and is projected to reach US$75.7 billion by 2030. The robust compound annual growth rate (CAGR) of 5.8% provides a clear signal that nasal delivery represents a shift in how drugs could be administered in the near future.
Several converging factors are at play in driving the market value of nasal drug delivery:
- Versatility of the Nasal Route: The nasal cavity offers a unique gateway for drug delivery, enabling local effects such as decongestion or allergy relief, systemic absorption and, most intriguingly, direct access to the brain. This versatility is unmatched by most other non-invasive routes.
- Patient-Centric Solutions: The nasal route is needle-free, often pain-free, and can be self-administered making it especially attractive for chronic conditions or emergency situations.
- Technological Advancements: The past decade has seen a wave of innovation in nasal delivery devices. Modern devices are more precise, user-friendly, and capable of delivering both liquid and dry powder formulations with remarkable efficiency. With the rise of personalised medication, there is a significant amount of research taking place in nasal delivery devices, with many focussing on increased absorption.
- Rising Interest in Dry Powder Forms: Dry powder nasal products offer advantages in terms of stability, portability, and rapid onset of action, further expanding the potential applications of this route.
Nose to brain (N2B) drug delivery
FDA Approvals Lead the Way
Perhaps the most compelling evidence of the nasal route’s growing importance is the surge in FDA approvals for nasal products in recent years. Since 2019, we’ve seen a diverse array of therapies reach the market, including:
- 2019: BAQSIMI® (dry-powder glucagon) for severe hypoglycemia.
- 2023: SPONTANTM (liquid Vardenafil spray) for erectile dysfunction.
- 2023: ZAVPRETTM (liquid Zavegepant spray) for migraine.
- 2023: OPVEE®, KLOXXADO®, NARCAN (liquid sprays for opioid overdose).
- 2024: NEFFY® (liquid epinephrine spray) for anaphylaxis.
- 2024: FLUMIST® (first OTC self-administered flu vaccine).
- 2025: VALTOCO® (liquid diazepam spray) immediate treatment of cluster seizures.
- 2025: SPRAVATO® (liquid esketamine spray) at home treatment for resistant depression.
- 2025: BREKIYA (liquid dihydroergotamine mesylate spray) migraine, cluster headaches.
- 2025: ATZUMI (dry powder dihydroergotamine mesylate spray) acute migraine.
These approvals underscore the nasal route’s ability to deliver rapid, effective treatment for a wide range of conditions, with many focussing on emergency scenarios.
Nose-to-Brain Delivery: Unlocking New Therapeutic Frontiers
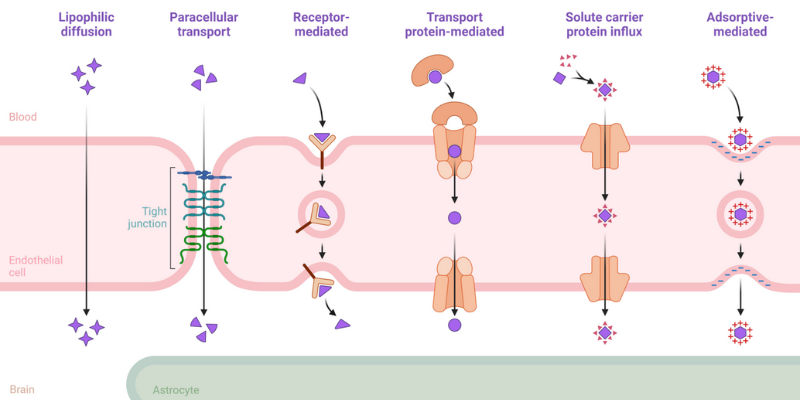
One of the most exciting frontiers in nasal drug delivery is the potential for nose-to-brain (N2B) delivery. The brain and central nervous system (CNS) represent some of the most challenging targets in medicine, largely due to the blood-brain barrier (BBB). This semi-permeable membrane is highly effective at protecting the brain from toxins, but it also blocks more than 98% of small-molecule drugs and virtually all large-molecule therapeutics.
Traditionally, only lipid-soluble molecules with a molecular weight under 600 Da could cross the BBB via passive diffusion. The BBB employs tight junctions, specific transport receptors, and efflux pumps to regulate what enters the brain, making drug delivery to the CNS a formidable challenge.
The nasal route offers a unique solution. By targeting the olfactory and trigeminal nerve pathways, drugs can bypass the BBB entirely, reaching the brain directly from the nasal cavity. This opens up transformative possibilities for treating:
- Pain management
- Neurodegenerative diseases such as Alzheimer’s, ALS, and Parkinson’s
- Brain cancers like neuroblastoma
- Acute CNS conditions such as seizures or migraines
As our understanding of the N2B pathway deepens, with growing understanding of the use of permeation enhancers and other excipients which can enhance delivery, and as device technology continues to advance, we’re likely to see even more innovative therapies leveraging this route in the coming years.
The Road Ahead: Innovation, Opportunity, and Patient Impact
The surge in nasal drug approvals since 2019 is more than just a regulatory trend—it’s a reflection of changing patient needs, scientific breakthroughs, and a collective drive toward more accessible, effective therapies. At Upperton, we’re proud to be at the forefront of this movement, partnering with innovators to develop and manufacture cutting-edge nasal formulations.
Looking ahead, I believe we’ll see:
- Continued growth in nasal product approvals, especially for CNS and emergency indications.
- Emergence of new device technologies capable of targeting the olfactory region with even greater precision.
- Expanded use of dry powder formulations for stability and rapid onset.
- Deeper scientific understanding of the N2B pathway, with greater knowledge of how different excipients can be leveraged to enhance delivery of a drug once it has landed on the olfactory epithelium, unlocking new therapeutic possibilities.