- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
What is a Finished Dosage Form?
What Does 'Finished Dosage Form' Mean in Pharmaceuticals?
A Finished Dosage Form (FDF) refers to a pharmaceutical product that has been processed and prepared into its final form for administration to patients. This includes tablets, capsules, injectables, creams, and other delivery systems that contain the active pharmaceutical ingredient (API) along with necessary excipients.
In simpler terms, the FDF is the version of the drug that is packaged, labeled, and ready for distribution and use. It is the culmination of formulation development, manufacturing, and quality control processes.


Finished Dosage Form (FDF) vs. Other Drug Components
To understand the importance of FDFs, it’s essential to distinguish them from other components in the drug development process:
- API (Active Pharmaceutical Ingredient): The biologically active component responsible for the intended therapeutic effect.
- Excipients: Inactive substances used to aid in the processing, stability, and delivery of the API.
- FDF (Finished Dosage Form): The final product that combines the API and excipients into a usable form (e.g., tablet, injection).
While APIs are the core of a drug’s therapeutic action, FDFs ensure that the drug is safe, effective, and convenient for patient use.
Common Types of Finished Dosage Forms in the Market
Finished dosage forms come in a variety of types, depending on the route of administration and therapeutic need. Common examples include:
- Oral Dosage Forms: Tablets, capsules, syrups
- Injectables: Vials, prefilled syringes, ampoules
- Topical Forms: Creams, ointments, gels
- Inhalation Products: Inhalers, nebulizers
- Transdermal Patches: For sustained drug delivery through the skin
Each form requires specific formulation and manufacturing techniques to ensure bioavailability, stability, and patient compliance.

Route of Administration
Common Types of FDFs
Oral
Oral
Injectable
Injectable
Topical
Topical
Inhalation
Inhalation
Transdermal
Patches
Key Guidelines for the Manufacture of Finished Dosage Forms
The manufacture of the finished dosage form is governed by strict guidelines to ensure consistency, safety, and efficacy. Key elements include:
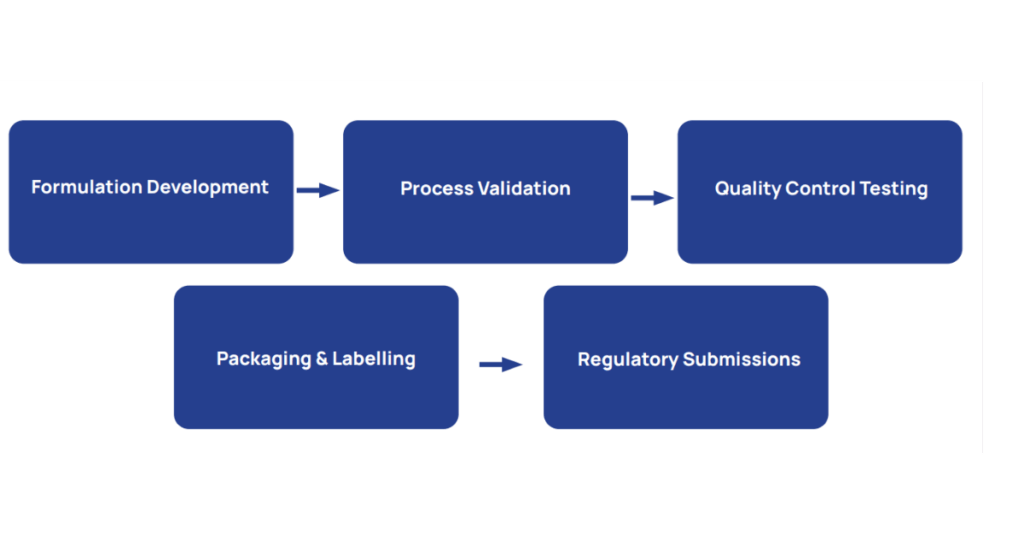
- Formulation Development: Selecting the right combination of API and excipients.
- Process Validation: Ensuring that manufacturing processes consistently produce quality products.
- Quality Control Testing: Verifying identity, strength, purity, and performance.
- Packaging and Labeling: Protecting the product and providing essential usage information.
- Regulatory Submissions: Documenting all processes and controls for approval by health authorities.
Guidelines such as the ICH Q8-Q10 series and FDA’s cGMP regulations provide frameworks for these activities.
Finished Dosage Form Manufacturing Process
Challenges in Finished Dosage Form Manufacturing
Despite technological advancements, FDF manufacturing presents several challenges:
- API Stability: Some APIs degrade easily and require protective formulations.
- Complex Formulations: Controlled-release or combination drugs require advanced technologies.
- Regulatory Hurdles: Meeting global compliance standards can be resource-intensive.
- Supply Chain Management: Ensuring consistent availability of raw materials and packaging components.
- Scalability: Transitioning from lab-scale to commercial-scale production without compromising quality.
Overcoming these challenges requires cross-functional expertise, robust quality systems, and often, collaboration with CDMOs (Contract Development and Manufacturing Organizations).
Frequently Asked Questions
What is the difference between an API and a Finished Dosage Form (FDF)?
An API is the active ingredient that provides therapeutic effects, while an FDF is the final product that includes the API and excipients, ready for patient use.
Why are excipients important in a Finished Dosage Form?
Excipients help stabilize the API, improve drug delivery, enhance taste or appearance, and ensure the product is safe and effective for administration.
What are some examples of Finished Dosage Forms?
Common FDFs include tablets, capsules, syrups, injectables, creams, inhalers, and transdermal patches.