- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Investigations into the Relationship Between Spray Dried Powder Particle Size and Deposition in Nose and Lung Analogues when Actuated from a Nasal Device
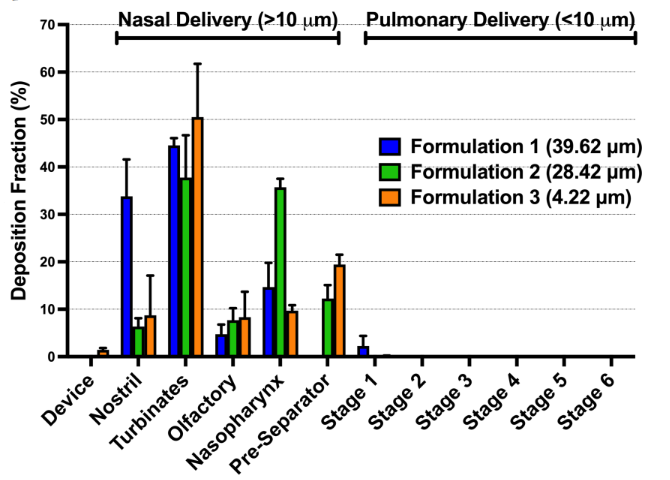
This scientific poster seminar will explore the relationship between spray dried powder particle size and deposition in nose and lung analogues when actuated from a nasal device.
Poster Overview:
The administration of therapeutics by nasal delivery is a growing area of pharmaceutical
development, due to the ease of administration and large, vascularised surface area available in
the nostril. However, there is a risk of pulmonary administration if powders are within the
respirable range. Inhalable particles are defined by several industries as those with a particle
diameter of 10 µm or less [1] , however the method of defining powder particle diameter is not
clear. There is currently limited pharmacopeial guidance on the testing of nasal powders
delivered from single dose nasal device.
Whilst laser diffraction is commonly used by the pharmaceutical industry to define particle
diameter and included as part of target product profiles for nasal products, a more biorelevant
test method is required that evaluates the combination of formulation and device.
Aims:
In this study, we release spray-dried powders with varying diameters (3.5 to 40 µm, measured
by laser diffraction) from an Aptar Unidose nasal powder device into an Alberta Idealized
Nasal Inlet (AINI), which simulates the nose [2] , connected to a Next Generation Impactor
(NGI) for lung simulation. The goal is to evaluate if laser diffraction is suitable for testing nasal
powders or if a more physiologically relevant method is needed
Meet our experts
Jordan Potts
Development Scientist
Lara Penn
Senior Development Scientist II
Jasmine Ahad
Senior Development Scientist I
Valentina Signorelli
Analytical Development Scientist I
Shailesh Mistry
Development Manager
Laura Mason
Director of Pharmaceutical Sciences
Download poster
Browse related articles