- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
A Thermofluor Screen for the Rational Formulation of Injectable Therapeutic Protein Solutions
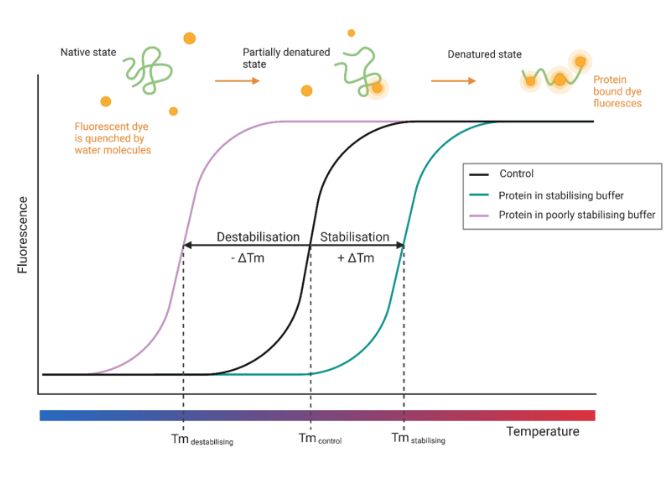
This scientific poster seminar will explore the use of a high-throughput thermofluor screen to inform rational formulation strategies for injectable therapeutic protein solutions.
Key Message
A 96-well thermofluor assay was developed to rapidly evaluate buffer systems for protein stability in therapeutic formulations. This approach enabled the identification of stabilising excipient combinations for a monoclonal antibody, with validation by dynamic light scattering confirming reduced aggregation. The optimised buffer demonstrated significant improvements in thermal stability and resistance to aggregation compared to water alone.
Introduction
Therapeutic proteins are inherently prone to denaturation and aggregation, which can compromise drug efficacy and safety. Injectable buffers play a critical role in stabilising proteins by enhancing non-covalent interactions and protecting against environmental and chemical stressors. However, conventional formulation screening methods such as buffer exchange or circular dichroism are resource-intensive and limited in throughput.
Thermal shift fluorimetry (thermofluor) offers a rapid, low-cost, and low-volume alternative for assessing protein stability. By monitoring changes in protein fluorescence during controlled heating, this technique enables the identification of buffering conditions that increase protein thermal stability (higher melt temperature, Tm).
Aim
To investigate the suitability of a 96-well thermofluor assay as a high-throughput tool for the rational optimisation of injectable protein formulations, and to validate an optimised buffer system for monoclonal antibody stability using dynamic light scattering (DLS).
Meet our experts

Jacob Poxten
Associate Development Scientist

Jordan Potts
Senior Development Scientist I