Nasal drug delivery route for small molecules and biologics.
Recent trends have seen growing interest in exploiting nasal drug delivery as a means of delivering a wide range of drugs including small molecules and larger biologics for a wide range of indications.
The nasal cavity is already a well-established delivery route capable of achieving rapid and high level of drug absorption, helped by the fact that it has a large surface area, with a porous endothelial membrane with high total blood flow. Add in the benefit of avoiding first pass metabolism and the ability to self administer helps make the nasal route an attractive proposition for a number of molecules. Intended delivery objectives could be
- Local delivery to the nasal membranes (an ideal target for vaccines, antivirals and prophylactics)
- Systemic delivery by absorption into the bloodstream (achieving fast action whilst avoiding first pass metabolism)
- Direct delivery into the brain (transcending the blood brain barrier for treatment of neurodegenerative and psychological disorders)
Nasal drug delivery devices
Traditionally developers are often met with a choice of two basic options; delivery from a device as either a liquid or dry powder formulation. Liquid formulations offer a number of benefits to the practitioner, usually in terms of speed, multi-dose options (for chronic use) and wider range of devices and ultimately cost. However, despite these advantages the dry powder equivalent devices are attracting growing interest in some key applications.

Benefits of dry powder nasal delivery
Whilst liquid formulations for nasal delivery are usually seen as the most cost effective and widely used approaches, there are some key differentiators /advantages for dry powder delivery. These key advantages for dry powder over liquid can be summarised as follows:
- No preservative required (powder formulations will not support microbial growth)
- Enhanced stability over liquid formulations (important for biologics and other unstable molecules)
- Improved delivery and retention in the upper regions of the nose (target area for direct delivery from nose to brain)
Improved shelf life/stability of dry powder formulations
Many APIs and biologics in particular are unstable in water and have to be stored frozen or refrigerated to protect the molecule from hydrolysis or aggregation. In addition many aqueous pharmaceutical formulations will support microbial growth, necessitating the need for a preservative which can pose regulatory challenges. One of the best ways of improving stability is to remove water from the formulation, using traditional drying technologies such as spray drying and freeze drying. Of these two approaches, spray drying has emerged as the technology of choice for developing dry powder formulations for nasal delivery as it has a number of benefits over freeze drying including the ability to engineer particles with the ideal size/aerodynamic properties to target the nasal epithelium whilst avoiding unwanted passage into the lungs.
Improved targeting for upper regions of the nose (to achieve direct to brain delivery)
Whilst systemic and local delivery of drugs via the nasal route is attracting increasing attention there is also growing interest in delivering drugs directly to the brain. Target diseases include the treatment of a wide range of CNS disorders including Parkinsons Disease, Multiple Sclerosis, Alzheimers, Epilepsy and Psychiatric disorders. Whilst there is a strong medical need for delivering drugs to the brain, researchers face a major challenge in the difficulties posed in getting their drugs across the Blood Brain Barrier (BBB), a physiological barrier that restricts the delivery of drug to the Central Nervous System (CNS).
Nasal delivery is now recognised as one of the best delivery routes for achieving delivery to the brain (and overcoming the BBB) by exploiting the direct transport pathway through the olfactory and trigeminal nerve cells, allowing drugs to bypass the BBB and enter brain directly. However, to achieve efficient nose to brain delivery it is important that the delivery of the administered dose provides consistent delivery to the correct regions in the nose to achieve delivery across the BBB. Imaging studies have shown that dry powder formulations can achieve superior distribution in these key olfactory regions compared to liquid devices/formulations as depicted in Figure 2 below:
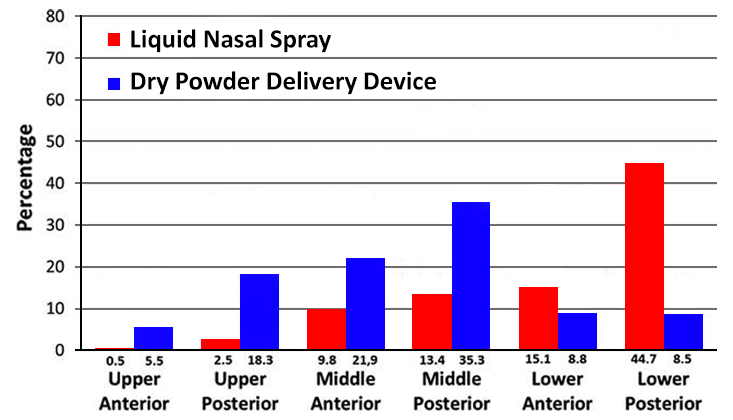
(per nasal region 0–2 min after administration)
(Adapted from Djupesland and Skretting (2012) journal of Aerosol Medicine and Pulmonary Drug Delivery vol. 25 no. 5)
As can be seen in the distribution profile shown, significantly more of the radiolabelled markers used was found in the six nasal regions 2 min after delivery of a dry powder formulation compared to a liquid spray device. The main explanation for this difference was loss of drug (radioactivity) due to anterior drip-out and to the pharynx within the first 2 min. Indeed, a higher fraction of the initial deposition was found in the anterior regions with powder delivery compared to the liquid spray (36 vs. 25%) More interestingly, in the upper posterior region of the nose (representing the key areas for targeting the olfactory regions necessary for nose to brain delivery) 18.3% of the initial deposition was observed for with the dry powder formulation compared with 2.4% for the traditional liquid spray).
Conclusions
There is increasing interest in developing dry powder nasal delivery formulations suitable for nasal delivery. Whilst liquid nasal sprays offer advantages in terms of cost and the options for multi dose devices. Dry powder delivery devices offer improved stability profiles and the potential for more efficient nose to brain delivery, key advantages for researchers seeking to develop nasal formulations containing unstable molecules such as vaccines, biologics and drugs targeting nose to brain delivery.
Nose to Brain Drug Delivery Whitepaper

Download our whitepaper that explores Nose to Brain (N2B) Drug Delivery, written by, Richard Johnson (Chief Scientific Officer).