- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Development and Activity Testing of Spray Dried Phosphorylated Hexaacyl Disaccharide for Nasal Administration
Jasmine Ahad1, Jack Sorrell1, Laura Mason1, Kristine Storey2 & Joe Ninosky2
1Upperton Pharma Solutions, Albert Einstein Centre, Nottingham Science and Technology Park, Nottingham, NG7 2TN, UK
2Revelation Biosciences Inc., 11011 Torreyana Road, Suite 102, San Diego, CA 92121, USA
Key Message
Spray dried PHAD formulation has been developed for administration as a micellar nasal spray to treat viral respiratory infections and allergies. Investigations found that PHAD concentration within spray drying feed solutions influenced the reconstitution properties of the resultant powder formulations, with reconstitution time, solution turbidity and micelle uniformity affected.
1. Introduction
Phosphorylated Hexaacyl Disaccharide (PHAD) is a synthetic structural analogue of Monophosphoryl Lipid A (MPLA), a phospholipid with immunostimulatory activity [1]. When in micellar form, it activates Toll-like receptor 4 (TLR4), leading to the production of protective cytokines and chemokines, and activation of CD4+ and CD8+ T-cells [1,2,3].
It is believed that PHAD must be in particle form, like a micelle, for best recognition and binding to TLR4, likely
as the micellar shape mimics a globular protein [4].
Micelles can be prepared by wetting PHAD with a solvent and adding water. However, liquid solutions have
shown poor solubility and high irritation on administration due to the presence of the non-aqueous solvent.
2. Aim
To spray dry PHAD in its micellar form to produce a powder formulation that would reconstitute in water back to its original micellar form and produce an immune response.
3. Experimental Methods
Michellar Feed Solution Preparation
Preparation of Micellar Feed Solution for the Spray Drying of 2:49:49 (%w/w/w) PHAD:HP-β-CD:Trehalose Formulation
- Prepared at PHAD concentrations varying from 0.3-1.0 mg/mL. Due to poor solubility of PHAD in water, higher
ethanol contents were required for increased PHAD concentrations (Table 1). - PHAD was sonicated at 40°C in 95% ethanol to produce a turbid nanosuspension.
- Excipient solution of Hydroxypropyl-β-Cyclodextrin (HP-β-CD) and Trehalose Dihydrate (Trehalose) in Water for
Injection (WFI) was heated to 40°C and added to the ethanolic PHAD at 10 g/min while sonicating at 40°C. - Following addition, the micellar feed solution was further sonicated at 40°C for 15 minutes and then
equilibrated to room temperature.
Table 1: Composition of spray drying feed solutions at varying PHAD concentrations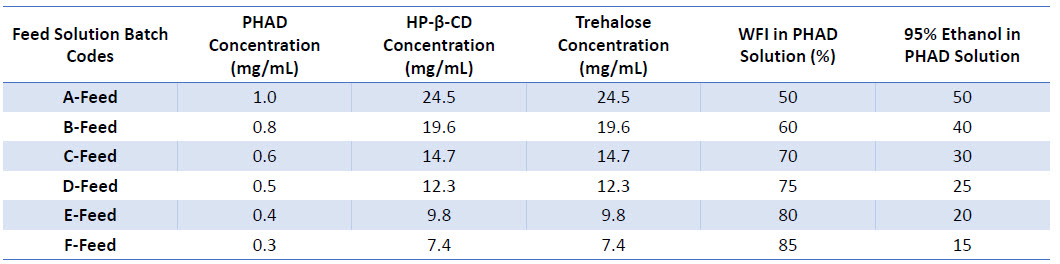
Spray Drying
Spray Drying of Micellar Feed Solutions
- Micellar feed solutions were spray dried using the ProCepT 4M8-Trix Spray Dryer with a two-fluid nozzle (0.8mm tip), using target spray drying parameters outlined in Table 2.
- Formulations were produced as white cohesive powders in 70-80% yield.
Table 2: Target Spray Drying Parameters for PHAD Feed Solutions

Reconstitution
Reconstitution of Spray Dried PHAD Formulations and Analysis of Micelles by Dynamic Light Scattering (DLS)
- Spray dried PHAD formulations were reconstituted in triplicate at 0.6 mg/mL PHAD in WFI with light vortexing.
- Dissolution time and visual observations of the resultant dispersion were recorded.
- Dispersions were analysed via DLS with backscatter at 20°C.
In Vitro Analysis
In Vitro Comparison of Liquid and Spray Dried PHAD Formulations
- Murine J774A.1 macrophages were cultured in DMEM medium containing 10% foetal bovine serum.
- 100K cells were plated per well of a 24-well plate and treated with 50 ng/mL PHAD in a liquid solution (ethanol
in water) or spray dried and reconstituted in water. - Cells were harvested 24 hours after seeding and pelleted by centrifugation at 250 x g.
- Supernatant was collected and centrifugation at 250 x g was performed to remove any residual cells.
- Supernatant then analysed per manufacturer’s protocols for the presence of IP-10 or TNFɑ using Invitrogen
and R&D Systems ELISA kits.
4. Results and Discussion
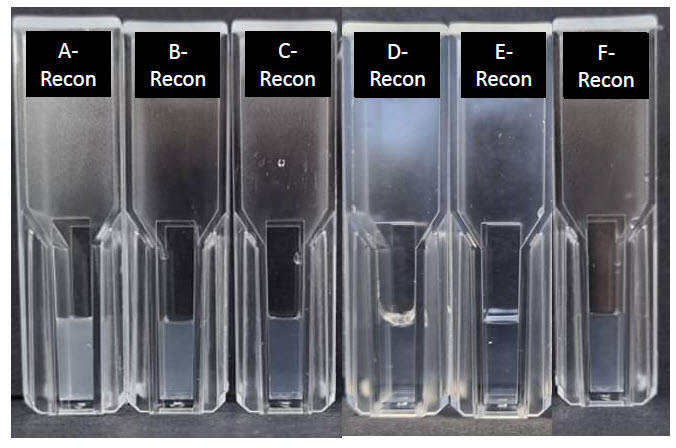
Figure 1: Spray dried PHAD formulations reconstituted at 0.6 mg/mL PHAD in WFI. Reconstituted batch codes correspond to feed solution batch codes outlined in Table 1
Turbidity of the reconstituted spray dried PHAD decreased with decreasing PHAD concentration within the feed solution (Figure 1). Decreasing initial PHAD concentration also increased ease of dissolution when reconstituting samples (Table 3).
Table 3: Summary of feed solution batches, their reconstitution times and micelle size distributions
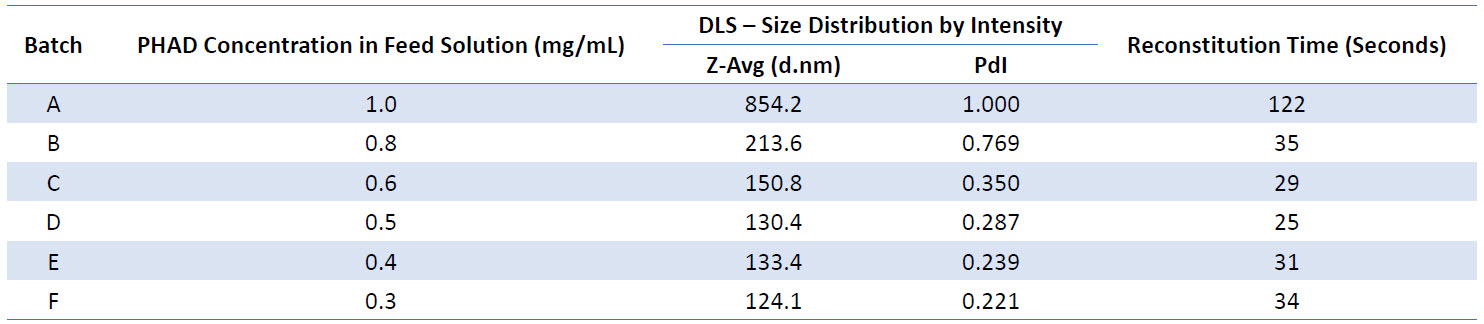
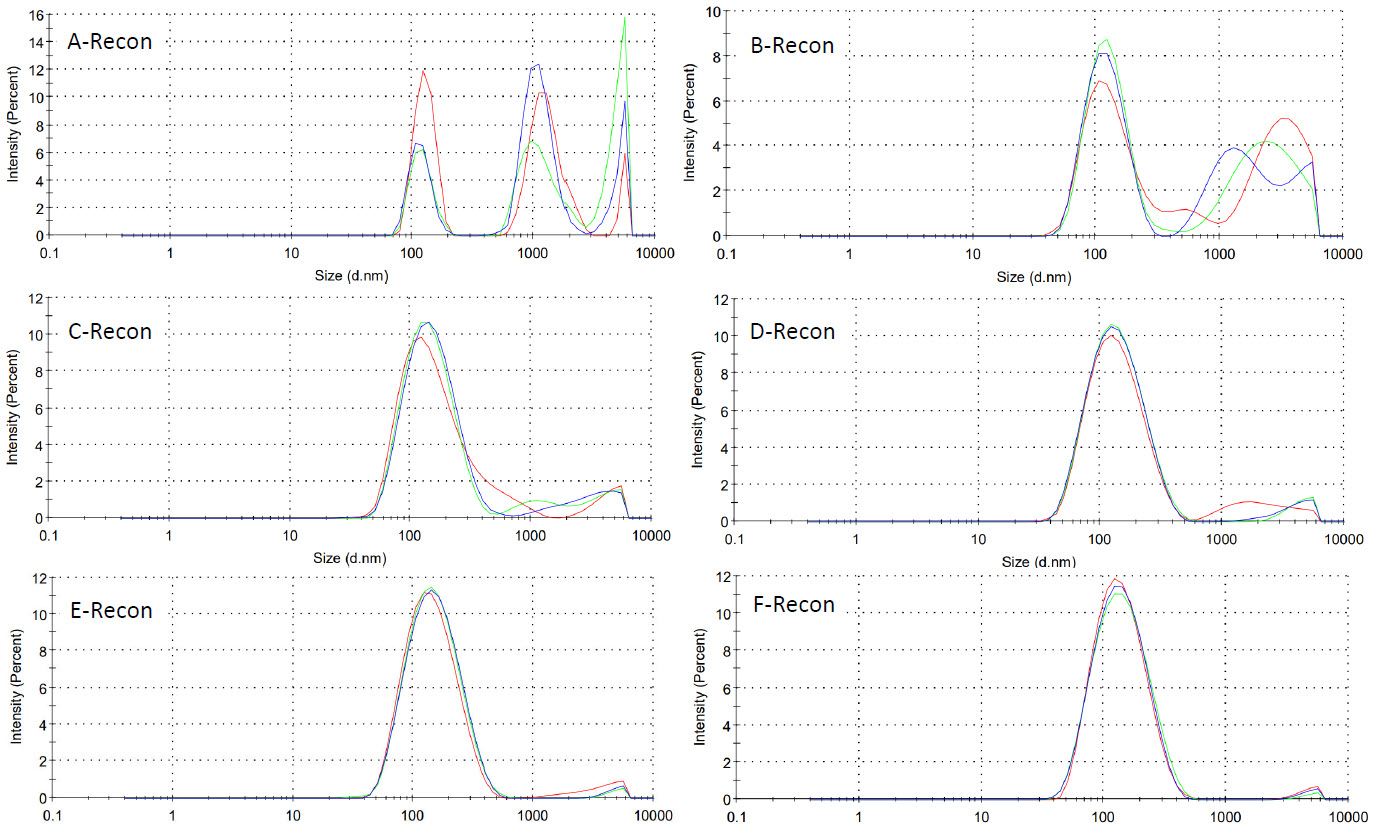
Figure 2: Size distribution traces of micelles formed from spray dried PHAD formulations reconstituted at 0.6mg/mL PHAD in WFI (3 average intensity measurements per batch)
Micelles within formulations spray dried from lower concentration feed solutions appeared more monodisperse, with lower PdI values observed (Table 3 and Figure 2).
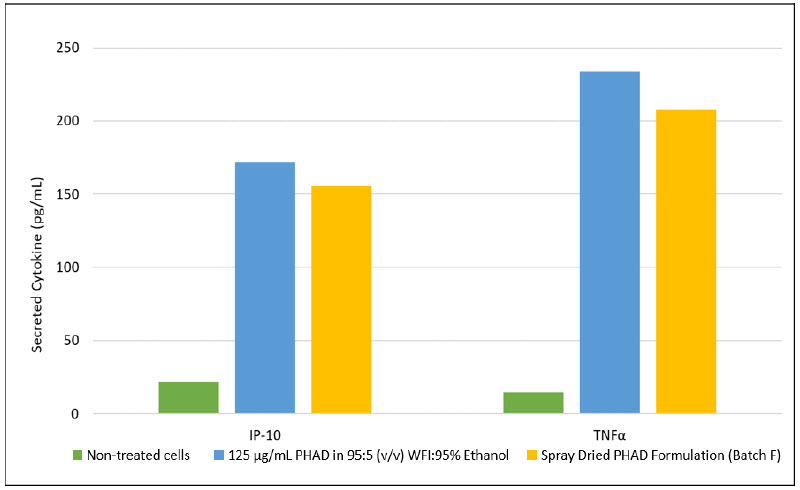
Figure 3: Levels of IP-10 and TNFα following 24-hour treatment of J774A.1 macrophages with 50 ng/mL of a 125 µg/mL PHAD in 95:5 WFI:95% Ethanol (v/v) and spray dried PHAD formulation (Batch F) (n=1)
In vitro activity was maintained after spray drying and mediated similar levels cytokine of production following treatment as PHAD in an ethanol solution (Figure 3).
5. Conclusion
- Decreasing the initial PHAD concentration resulted in:
- Reduced reconstitution times
- Decrease in turbidity of the micellar solutions.
- Uniform micelles – monomodal in size, with lower PdI values.
- 0.5 mg/mL PHAD showed to be the most effective in maintaining the balance of spray drying processing times without compromising the integrity of the reconstituted micelles.
- Reconstituted solutions maintained in vitro activity, being able to produce a cytokine response comparable to the original PHAD in ethanolic solution.
- The powder will be further investigated as a stable form of PHAD that can be administered intranasally, either as a reconstituted solution or as a powder
References
[1] National Cancer Institute. (2022, July 12). Definition of monophosphoryl lipid A – NCI Drug Dictionary – NCI. National Cancer
Institute. Retrieved July 12, 2022, from
https://www.cancer.gov/publications/dictionaries/cancer-
drug/def/monophosphoryl-lipid-a
Casella, C. R., and Mitchell, T. C. (2008, August). Putting endotoxin to work
for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant.
Cellular and Molecular Life Sciences, 65, 3231 – 3240. DOI: 10.1007/s00018-008-8228-6
[3] Kamphuis, T., Meijerhof, T., Stegmann, T., Lederhofer, J., Wilschut, J., and de Haan, A. (2012, May). Immunogenicity and Protective Capacity of a Virosomal Respiratory Syncytial Virus Vaccine Adjuvanted with Monophosphoryl Lipid A in Mice. PLOS ONE, 7(5), e36812. DOI:
10.1371/journal.pone.0036812
[4] Ho Min, K., Beom Seok, P., Jung-In, K., Judong, L., Se Cheol, O., Purevjav, E., Norio, M., Hayyoung, L., Ook Joon, Y., and Jie-Oh, L. (2007, September
7). Crystal Structure of the TLR4-MD-2 Complex with Bound Endotoxin Antagonist Eritoran. Cell, 130 (5), 906-917. DOI: 10.1016/j.cell.2007.08.002