- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Investigations into Spray Dried Powder Particle Size and Deposition in Nose and Lung Analogues when Actuated from a Nasal Device
Jordan C. Potts1, Lara C. Penn1, Jasmine Ahad1, Valentina Signorelli1, Tom J. Jepras1, Shailesh K. Mistry1, Laura M. Mason1
1. background
The administration of therapeutics by nasal delivery is a growing area of pharmaceutical development, due to the ease of administration and large, vascularised surface area available in the nostril. However, there is a risk of pulmonary administration if powders are within the respirable range. Inhalable particles are defined by several industries as those with a particle diameter of 10 μm or less [1] , however the method of defining powder particle diameter is not clear. There is currently limited pharmacopeial guidance on the testing of nasal powders delivered from single dose nasal device.
Whilst laser diffraction is commonly used by the pharmaceutical industry to define particle diameter and included as part of target product profiles for nasal products, a more biorelevant test method is required that evaluates the combination of formulation and device.
2. Aims
In this study, we release spray-dried powders with varying diameters (3.5 to 40 μm, measured by laser diffraction) from an Aptar Unidose nasal powder device into an Alberta Idealized Nasal Inlet (AINI), which simulates the nose [2] , connected to a Next Generation Impactor (NGI) for lung simulation. The goal is to evaluate if laser diffraction is suitable for testing nasal powders or if a more physiologically relevant method is needed.
3. Methods
Spray Dried Formulations:
Three solutions were spray dried using two different nozzles (ultrasonic and two-fluid) to create particles of varying sizes with HPMC, Mannitol, and Caffeine (Formulations 1 and 2), or Trehalose and Caffeine (Formulation 3).
Coating Solution & Equipment Assembly:
A coating solution was made with Brij-35, glycerol, and ethanol. The AINI and NGI collection cups were coated and assembled with a pre-separator containing 10 mL water between them.
Deposition Profile Testing:
The device, loaded with 20 ± 3 mg of formulation, was angled at 45° and inserted 1 cm into the AINI’s nostril. Airflow was applied for 15 seconds upon actuation. Afterward, the setup was disassembled and rinsed with 10 mL water, per stage, to dissolve powder deposits. Each formulation was tested in duplicate.
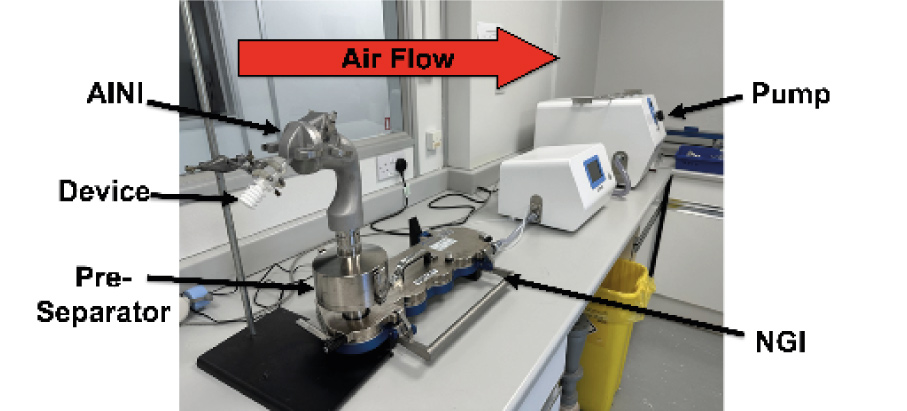
4. Results and Discussion
The main study compared particle diameter by laser diffraction to AINI nasal deposition in Figure 2 and Table 1.
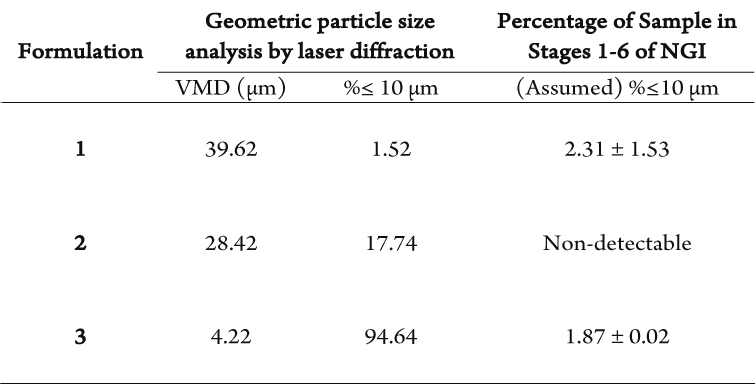
- Formulation 1: 1.52% respirable particles, similar to NGI deposition
- Formulations 2 and 3: 17.74% and 94.64% respirable particles, minimal NGI deposition
- Deposition profiles mainly in nasal area (AINI)
- Plume geometry and particle density may influence deposition more than particle diameter alone
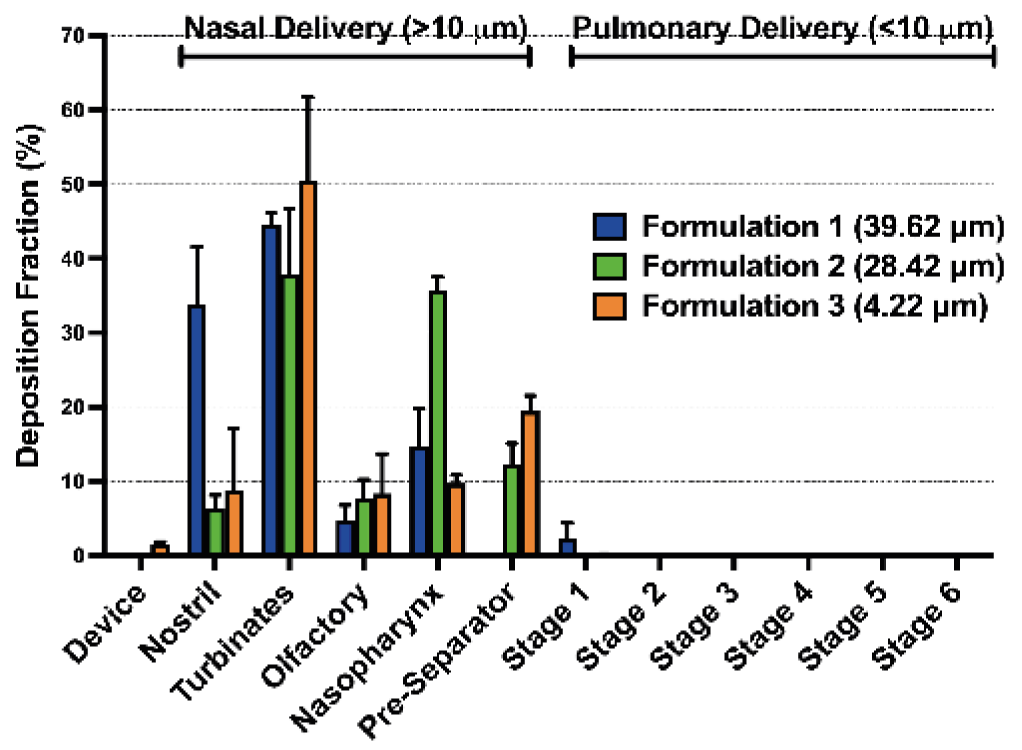
Figure 2: Deposition fraction of spray dried powders of 4.22 µm, 28.42 µm and 39.62 µm volume mean diameters in a coated AINI and NGI.
The correlation between geometric particle diameter and nasal deposition was investigated by producing 3 spray dried formulations with varying particle diameter and therefore, differing percentage of particles with a diameter of <10 µm (Table 1).
Table 1: Comparison of percentage of particles with a diameter less than 10 µm analysed geometrically by laser diffraction and aerodynamically by NGI deposition of spray dried material. Volume mean diameter = VMD.
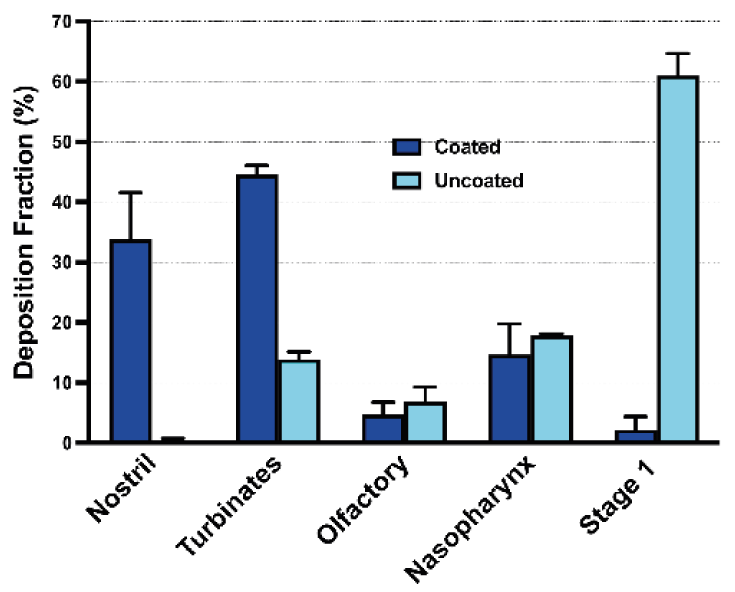
The coating’s effectiveness was evaluated by comparing the deposition profile of Formulation 1 in coated and uncoated AINI when released from a device (Figure 3):
- Formulation 1: VMD 39.62 μm, 1.52% particles <10 μm, expected minimal NGI deposition
- Coated AINI: significant profile change, most powder in AINI, only 2.31 ± 1.53% in NGI
- Uncoated AINI: over 60% powder in Stage 1, not suitable for comparing to in vivo nasal environments
5. Conclusion
In summary, we examined the connection between spray-dried powder particle diameter and nasal deposition from an active device. Particle diameter had a smaller impact on nose deposition profiles than expected. Our findings suggest that the guideline of “no more than 10% of particles with a diameter less than 10 µm” may not be as crucial as previously thought, particularly when using the tested device. Aerodynamic particle diameter measurements with the intended device are needed to understand the respirable fraction and set development targets. More collaboration with regulatory agencies is required to establish the best testing practices for single-dose nasal powders.
6. References
1. Meng X, Wu Y, Pan Z, et al. Seasonal characteristics and particle-size distributions of particulate air pollutants in Urumqi. Int J Environ Res Public Health 2019;16. doi:10.3390/ijerph16030396
2. Kiaee M, Wachtel H, Noga ML, et al. An idealized geometry that mimics average nasal spray deposition in adults: A computational study. Comput Biol Med 2019;107:206–17. doi:10.1016/j.compbiomed.2019.02.013