- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
UpperNoseTM Fact Sheet
For successful nasal delivery of small molecules and biologics
The ability to self-administer small molecules, biologics and vaccines is highly attractive yet the knowledge and expertise necessary to rapidly develop products for this delivery route remains limited in the CDMO space.
To meet this growing demand, Upperton has developed UpperNoseTM a unique nasal product development platform. Our aim is to make it easier and faster for small and mid-sized innovators to access the knowledge and capabilities necessary for the successful development of nasal dosage forms.
The UpperNoseTM platform can be applied to liquid and dry powder formulations and utilises the knowledge and expertise of the highly experienced Upperton scientific team to develop your molecule in an efficient and timely manner.
Applications
Local delivery to the nasal membranes (e.g. for vaccines, antivirals, prophylactics)
Systemic delivery by absorption into the bloodstream (e.g. for fast action whilst avoiding first pass metabolism)
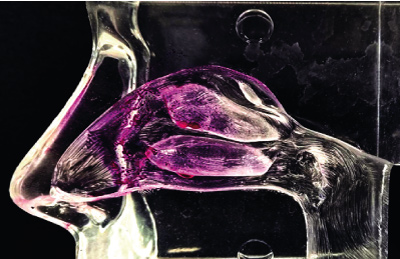
Direct delivery into the brain (transcending the blood brain barrier for treatment of neurodegenerative and psychological disorders)
Working closely with our clients, we will quickly identify the most time/cost effective development programme to facilitate early, smooth passage of our customer’s molecule from early-stage development into clinical trials.
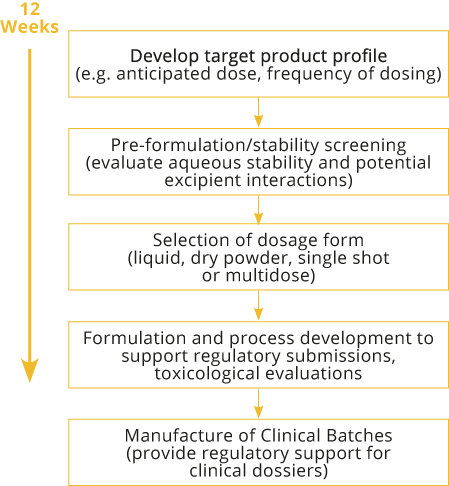
Typically we will take our customers through a series of well-established development steps although in reality, some of these can be combined or omitted depending on the dosage form required.

The UpperNoseTM Development Pathway

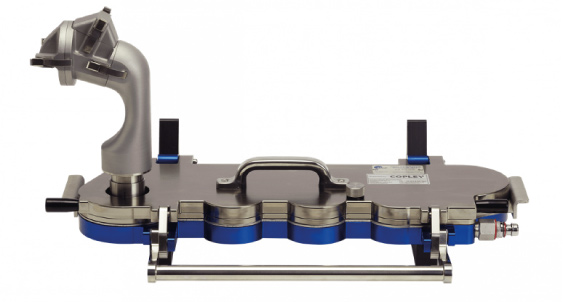
Copley AINI device attached to the Next Generation Impactor (NGI)