- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Choosing Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Technical Services Fact Sheet
We Offer Our Clients an Extensive Range of Services, From Early Preformulation/Feasibility Studies, Right Through to Dosage Form Development and Clinical Trial Manufacturing
Our Business
Upperton Pharma Solutions is an early phase Pharmaceutical Contract Development and Manufacturing Organisation (CDMO) with over twenty years of experience in the development of liquid and dry powder pharmaceutical dosage forms.
Upperton’s expertise covers a wide range of pharmaceutical technologies, from traditional powder blending, granulation, tabletting and capsule filling to more advanced enabling technologies such as spray drying and jet milling, for bioavailability enhancement, and particle engineering for targeted pulmonary and nasal delivery.
Our Pharmaceutical Sciences team have extensive experience in formulating and delivering a
wide range of molecules including:
Small molecules (APIs)
Biologics (proteins,
peptides, vaccines)
Controlled drugs covered
by UK Home Office Licences
Schedules 1-4
Clinical trial manufacturing is undertaken in our MHRA licensed facility, and dosage forms include liquid solutions and suspensions, tablets, capsules and pulmonary/nasal devices. Products are tested in our licensed QC laboratories and released by our QPs for use in clinical trials around the world.
Handling Capabilities
In our ISO 8 clean room we handle a wide range of APIs; typically SafeBridge Category 1-3. However, by using our isolator technology we can formulate and fill more potent molecules on a case-by-case basis.
Analytical Services
Upperton’s pharmaceutical development and manufacturing services are underpinned by a comprehensive in-house analytical capability; from traditional chemical testing techniques such as HPLC, GC, UV and FTIR, to a more advanced solid-state analysis.
The physical and thermal properties of dry powder formulations are characterised using techniques including Dynamic Vapour Sorption (DVS), Differential Scanning Calorimetry (DSC), powder X-ray Diffraction (pXRD), Thermogravimetric Analysis (TGA), laser particle size analysis and Scanning Electron Microscopy (SEM).
Analytical methods are developed in-house and qualified to a phase-appropriate level by the Upperton QC team. These are used to guide product development, right through to QP release of clinical products and shelf life assignment based upon ICH stability studies. All data generated is made available to support regulatory submissions.
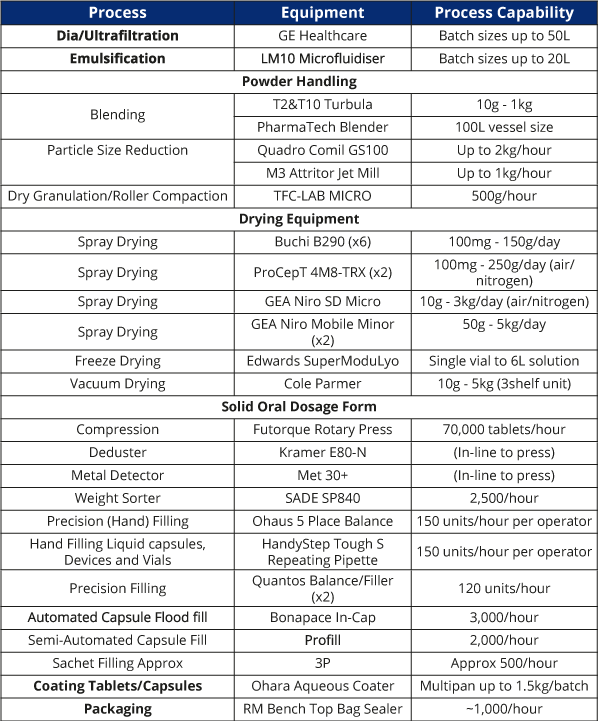
Key Pharmaceutical Processing Equipment (and Scale)