- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Oral Solid Dosage Forms Fact Sheet
Offering Expert Preclinical and Clinical Formulation Development of Oral Solid Dosage Forms
Oral administration remains the most widely used route for the successful delivery of active pharmaceuticals to patients. Dosage form design will be driven by a combination of factors
including:
- Speed of development (fast into clinic)
- Anticipated dose
- Excipients required to improve API stability or enhance bioavailability
- Targeted (enteric) or modified (delayed) release profile requirement
Developing Oral Dosage Forms
Upperton can offer clients expert preclinical and clinical formulation development of oral solid dosage (OSD) forms; from early feasibility, right through to clinical manufacture. Formulations can range from simple powder in bottle/sachet for reconstitution to more complex capsules and tablets.
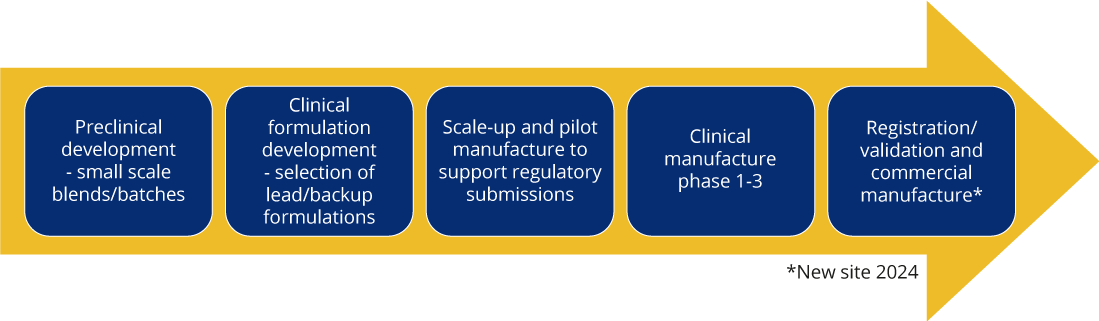
Typical Development Pathway
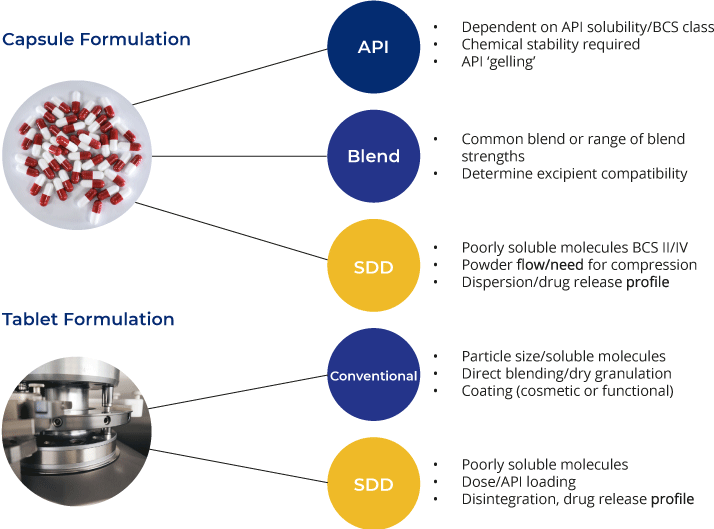
Capsule Formulations
Capsule formulations are often the favoured dosage form for initial clinical studies as they offer a fast, convenient way to deliver the API to the pharmacy. Capsules may contain simple unformulated API or more advanced formulations, such as amorphous spray-dried dispersions designed to enhance bioavailability.
Tablet Formulations
Whilst capsule delivery represents the faster route into the clinic, tablets give more flexibility in the formulation and offer a more cost-effective solution for products entering later stages of development or commercial manufacture, as well as being a more established approach for targeted delivery.
Analytical and Stability Testing
Phase-appropriate methods are developed for analysis of OSDs from early-stage development, through to GMP manufacture.
Characterisation of OSDs Typically Includes:
- Appearance – visual and
microscopic - Material identification
- FTIR
- Assay and related
substances – HPLC, UV, GC - Water content – Karl
Fischer - Residual solvents – GC
- Blend homogeneity
- Blend density/
compressibility - Content uniformity/
weight uniformity - Tablet hardness/
friability - USP disintegration and
dissolution - Discriminating/
clinically relevant
dissolution - Microbial limits
(outsourced)