- What we do
-
-
Routes of delivery
- Oral
- Nasal
- Nose to Brain
- Pulmonary
- Parenteral
-
Development stage
- Pre-clinical
- Phase I to Phase II
- Phase III - Commercial
Our Approach
- Selecting Your CDMO Partner
- Project Management
-
- About us
-
-
A CDMO like no other
- About Us
- Our Facilities
- Our History
- Awards and Achievements
-
Leadership Expertise
- Executive Leadership Team
- Board of Directors
- Careers
-
-
- Resources
- Events
- Contact
Expertise
Our Leadership team has extensive collective experience in steering products from pre-clinical to commercial manufacture.
Science-led
Our approach is scientific, collaborative and open, ensuring an open dialogue and transparency that fits your project.
Award-winning
We are a leading CDMO recognised for numerous awards including a King's Award For Enterprise.
Nimble
We adapt to your project needs, and pledge to never relegating it to the bottom of the list.
State-of-the art GMP manufacturing facility.
Our state-of-the-art Nottingham facility houses 10 GMP manufacturing suites, quality control laboratories and dedicated analytical and formulation development laboratories with pilot plant facilities.
Trent Gateway has been specifically designed to cater to a wide range of development and manufacturing projects, offering processing of non-sterile dosage forms including solids, liquids, semi-solids, nasal and inhaled products.
Whether you’re in the early stages of formulation development, in need of clinical trial supplies from Phase 1 to Phase 3 or seeking niche-scale commercial manufacturing, Trent Gateway is fully equipped to fulfill your requirements with precision and excellence.

Our facilities at a glance
sqft GMP facility
0
sqft warehouse
0
sqft analytical labs
0
sqft R&D laboratories
0
Research and Development
- 10,000 sqft laboratories
- Dosage form development up to 5Kg
- Pilot laboratories with containment
for potent processing - Dedicated analytical development team
- ASAP stability test suite

GMP Manufacturing
- 10 advanced GMP manufacturing suites
- Process trains supporting oral, pulmonary
and nasal dosage forms - Flexible manufacturing
- Sterile processing capability
- High potency containment
- Clinical packaging and labelling
- MHRA Approved
- Home Office Approved (Schedule 1 -4)
Quality Control & Analysis
- 8,000 sqft analytical laboratories
- Dedicated laboratories and staff
- Designated HPLC and dissolution laboratories
- Small molecule and biological test equipment
- Humidity controlled areas for moisture
sensitive products

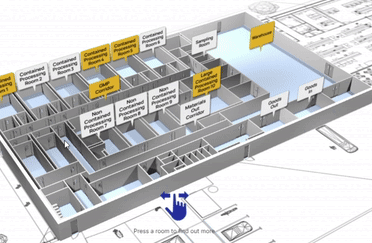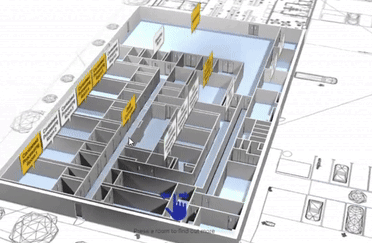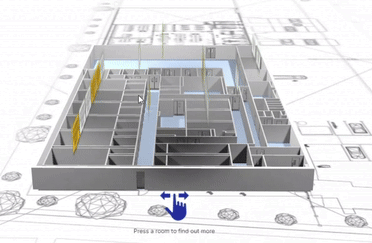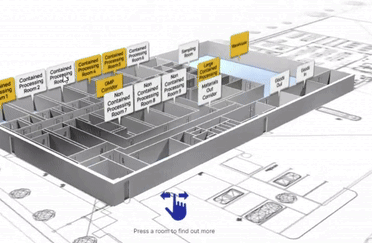
View our interactive facility tour.
Step inside our award-winning Trent Gateway facility and view our GMP processing rooms, R&D laboratories and dedicated QC space.