Introduction to Nasal Delivery
The delivery of APIs via nasal inhalation is of growing interest to companies looking to exploit the benefits of this delivery route.
Delivery targets are typically broken down into three categories
- Local delivery to the nasal membranes (e.g. for vaccines, antivirals, prophylactics)
- Systemic delivery by absorption into the bloodstream (e.g. for fast action whilst avoiding first pass metabolism)
- Direct delivery into the brain (transcending the blood brain barrier for treatment of neurodegenerative and psychological disorders)
The majority of nasally inhaled formulations are predominantly developed for liquid devices, either as one time use or a multiple use option. Whilst liquid formulations remain the most prevalent and widely used approach, companies are now considering dry powder delivery options for certain classes of molecules.
The benefits of dry powder formulations over conventional liquid nasal formulations
Typically, it will be the physicochemical properties of the API that will dictate which formulation approach will be used. Indeed, dry powder nasal formulations can offer the following advantages over liquid versions:
- greater product stability
- enhanced bioavailability
- potential for higher dosing
- delivery of molecules with low aqueous solubility
- Inclusion of permeation enhancers and bio adhesives at a molecular level
- Potential to target more difficult areas in the nasal cavity (eg the olfactory regions).
Engineering Particles with optimal size for nasal delivery
When choosing to use dry powder for nasal medication, it’s important to create particles of the right size for the device used. This is needed to ensure (1) the device achieves optimal performance, (2) the particles target the correct regions in the nasal cavity, and (3) to reduce the presence of particles in the respirable size range ie with the potential to pass through the nose and deposit in the deep lung.
Spray drying is now emerging as the best formulation technology for developing dry powder formulations that are optimally sized for nasal delivery.
To achieve the best device performance and nasal delivery, most experts aim for particles with an X50 size (the size where 50% of the particles are smaller and 50% are larger) between 20-50µm. This size is ideal for reaching the olfactory and turbinate regions of the nose.

Fig 1 Scanning Electron Micrograph of a spray dried nasal dry powder.
It’s also crucial, from a safety and a regulatory perspective, to minimize the number of small particles in the medication as these have the potential to travel through the nose and deposit in the deep lung. The particle size target that is often quoted is a figure of “less than 10% of the particles in the delivered dose should be below 10µm in size”
Methods for Determining Particle Size
Particle size analysis: laser diffraction
When developing spray-dried medications, it’s important to determine the size of the powder particles produced. This is done using laser diffraction with a particle size analyzer like the Sympatec®. The dry powder is spread over a laser beam, and the pattern helps determine the particle size distribution. A typical size distribution for a nasal spray powder can be seen in Figure 2. During the spray drying optimization process, it is usual to aim for particles where the X50 size is between 20-50µm, with less than 10% of particles (by mass) being below 10µm
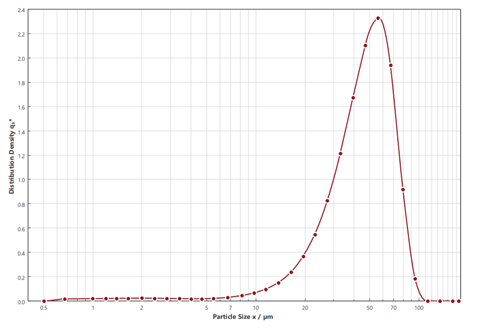
Fig 2 Laser diffraction particle size analysis (Sympatec®) of a nasal dry powder formulation
Aerodynamic particle size analysis: Alberta Idealised Nasal Inlet device (AINI)
Whilst determining geometric particle size distribution is an important tool for assessing formulations during the development phase, this technique does not measure the aerodynamic particle size of the powders.
The way particles move in a dry powder nasal dosage form depends on various factors. Although particle size affects where the drug will be deposited in the nose and lungs, other factors like the powder’s bulk density, device performance, and patient instructions can also influence the deposition site. These factors are important for safety because they affect how much of the dose ends up in the lungs.
New measurement techniques have been developed to determine where a dry powder inhaler formulation might deposit in the nasal airways/lungs. One such method, called the Alberta Idealised Nasal Inlet device (AINI), is increasingly used by practitioners.
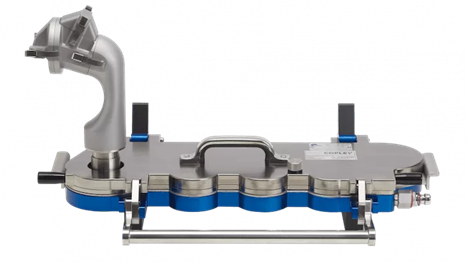
The AINI is an anatomical model of the nasal airways, developed at the University of Alberta and commercialised as a test device by Copley Instruments (Nottingham, UK). It was developed from a series of CT scans of 10 human nasal geometries and creating a “best fit” to represent a patient nasal airway geometric model. The AINI breaks down into four basic sections and enables the practitioner to asses drug deposition behaviour in each region, The AINI has a four-region resolution:
- the vestibule (nostril),
- the turbinates,
- the olfactory region,
- the nasopharynx.
The AINI is easily separated into its component parts (see Figure 3) to enable drug recovery and assay for each of the four individual areas after the dose of drug has been administered.
Fig 3 AINI broken down into four main compartments. - Whilst the distribution of the drug in the four regions of the nasal airways is important, the safety aspects also need to be addressed ie what is the potential for powder to deposit in the lung after nasal administration?
- To evaluate lung deposition the AINI can be attached to a Next Generation Impactor (NGI) so that the proportion of the delivered dose that ends up in the lungs (ie on the different stages of the NGI) can also be determined (see Figure 4)
Spray dried nasal formulations of different sizes can be filled into a nasal delivery device and delivered into a simulated nasal/lung system. Following delivery by the nasal device, the two impactors representing the nose (AINI) and the lung (NGI) can be opened, and drug deposition determined using a range of analytical techniques (e.g., HPLC, spectrophotometric methods).
Data generated from both impactors can then be used to calculate 1) where in the nose the drug will be deposited and 2) how much of the delivered dose might end up in the lung (crucial from the safety perspective).
Figure 5 shows the typical dose distribution of a spray-dried powder delivered using an Aptar UniDose® dry powder device in the test system depicted in Figure 4.
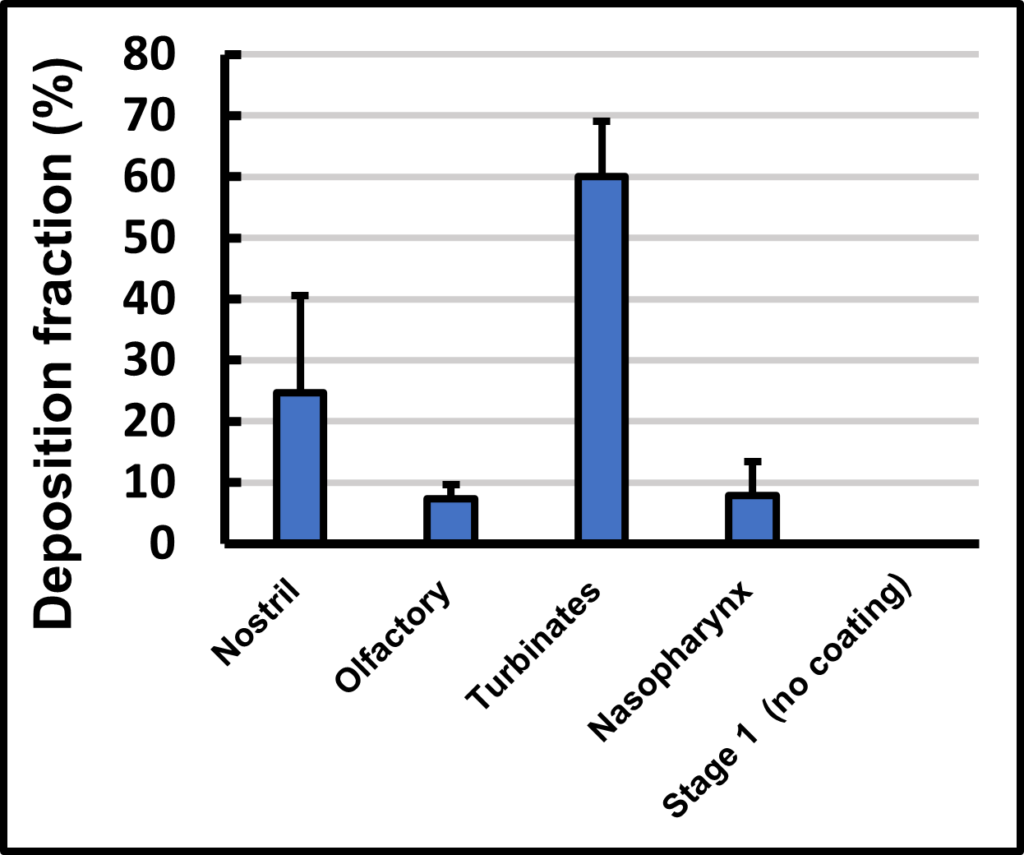
Fig 5 Nasal/Lung drug deposition of a spray dried powder delivered by an Aptar UniDose® device
Conclusions
Determining the particle size of spray dried powders is a crucial tool for the successful development of nasally inhaled dry powder dosage forms.
Laser diffraction particle size analysis is routinely used when optimising formulations and spray drying conditions to achieve particles in the 20-50µm particle size range. However, whilst this method offers a useful, relatively quick method for measuring particle size, it does not provide data on what proportion of the dose of powder delivered from a nasal device will deposit within the different compartments in the nasal passage and subsequently how much of the delivered dose might reach the lung.
The distribution of a delivered dose from a dry powder nasal device will ultimately depend on a number of factors including the geometric and aerodynamic particle size which in turn will be influenced by a number of factors such as powder properties (eg bulk density) and the device used to deliver the powder.
New nasal deposition test systems such as the AINI have been developed to shed more light on where a powder might deposit within the different regions of the nasal passage. In addition, when attached to the NGI, the test systems can also measure important safety aspects i.e. how much of the delivered dose might pass through the nasal passageway into the lung.
For further details or to discuss options for nasal delivery, contact Upperton Pharma Solutions and speak with one of our technical experts.