Overview of Nasal Delivery
58% of all therapeutics in development are injectable, 29% oral and 4% inhaled including nasal delivery, and while nasal delivery may not be one of the most explored routes, it holds the potential to offer an alternative delivery method for therapeutics with specific advantages.
The nasal cavity presents a large area of highly vascularised accessible mucosa. Delivery is non-invasive and avoids the first-pass hepatic metabolism. Treatments have a rapid onset of action in many cases; rendering it well suited for local treatments and potentially systemic.
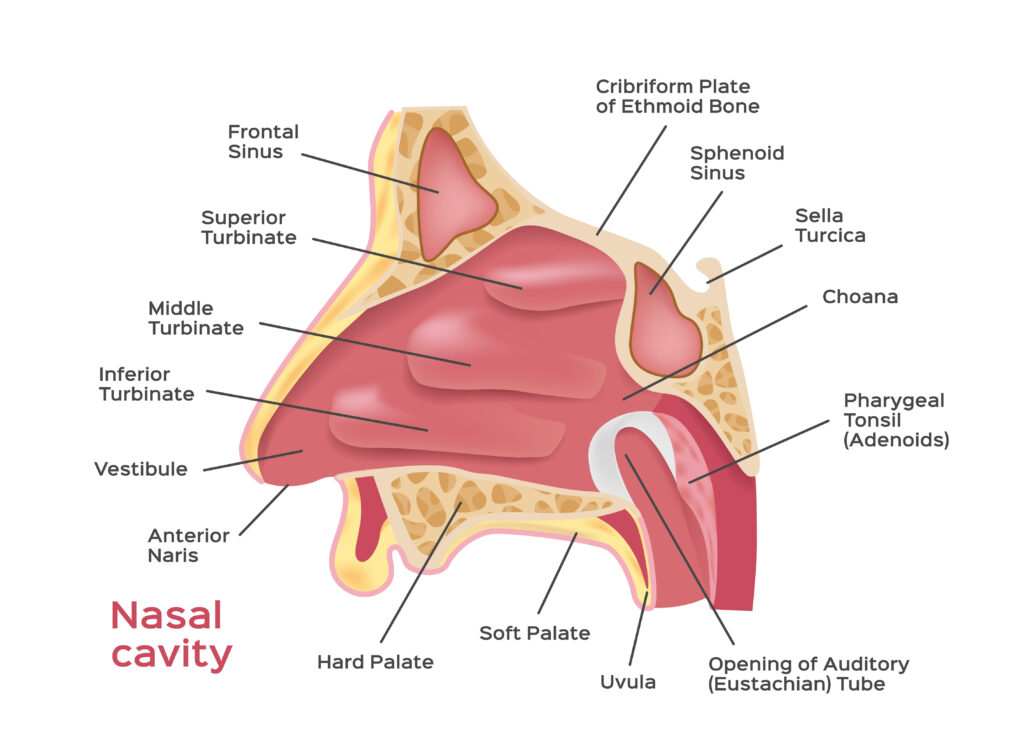
The nose is a complex organ. The anterior portion (the visible part) is largely lined with non-ciliated, squamous epithelial cells and is not the preferred site for drug delivery. For most systemic applications, the turbinates or concha, scroll-like features along the sides of the nasal passageways and covered with ciliated mucosal tissue are where absorption mainly takes place. The olfactory region, in the ‘roof’ of the nasal cavity, is of key interest for CNS delivery; nerve fibres from the olfactory bulb (within the frontal brain) extend through a thin, perforated portion of the skull (the cribriform plate) to present a high density of olfactory receptor cells within the nasal cavity. These form a connection from nose to brain, bypassing the blood-brain barrier.
Nasal delivery can be advantageous for its relative ease of use, patient acceptability and compliance and elimination of sharps. It is a suitable route for those compounds that are destroyed in gastrointestinal fluids if taken orally, are metabolised in the wall of the GI tract or undergo extensive biotransformation by the liver during their first passage in circulation. This article will talk through a few of the beneficial key applications and advantages of nasal delivery.
Local Delivery within the Nasal Cavity
The nose can often be a primary route of infection for many diseases or ailments such as coronavirus or influenza, or the site of allergies such as hay fever and rhinitis. Therefore, delivering therapeutics directly to the desired location of action can induce an immediate effect before the pathogen / allergen has an opportunity to be exposed within the body.
Nasal delivery has been proven to be effective in the delivery of peptides, polypeptides, vaccines and antisense DNA, which are traditionally administered via injection. Nasal delivery offers a feasible alternative to injected delivery route formulations. This is due to the large surface area, porous endothelial membrane, high total blood flow, the avoidance of first-pass metabolism and ready accessibility.
Nasal mucosal vaccines offer advantages over injectables, in the case of dry powder nasal vaccines there is no need for cold chain shipment and storage; rendering it a more cost-effective dosage form. The administration is simpler, requiring less training for the administrator / patient and is less invasive as it doesn’t require the use of hypodermic needles, which in itself can pose a risk of infection.
Local delivery within the nasal cavity is fast acting. However, nasal clearance can pose a problem as your drug (without mucoadhesives) has on average of 10 to 15 minutes to be absorbed into the cell lining (20 to 30 minutes with mucoadhesives).
Local delivery of therapeutic within the nose has been explored for nasal vaccines, hay fever and anti-rhinitis treatments.
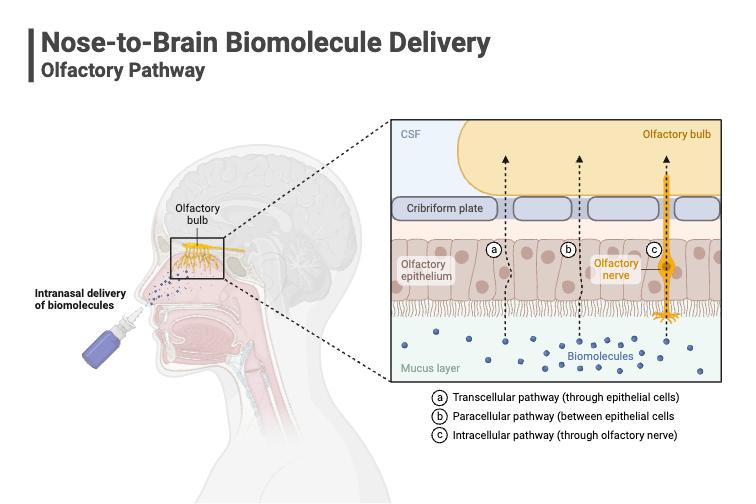
Delivering Therapeutics Directly to the Brain
By March 2020, there were 3057 treatments in development for neurological disorders, which is a 10% increase from 2019. The brain is the centre neurological activity within the body. Delivering therapeutics to the brain can prove to be a feat as the body has developed a natural defence with the blood-brain barrier. The olfactory region presenting a connection from the nose to the brain, bypassing the blood-brain barrier, is of keen interest of those developing therapeutics for neurological disorders.
The choice of device used in nasal delivery is critical as it needs to ensure the therapeutic reaches the olfactory region.
Fast-acting Absorption and Therapeutic Delivery
In the right circumstances nasal delivery can result in rapid systemic drug absorption. This is crucial for certain patient profiles / scenarios; for example, if a patient is unconscious then the need for a simple application and fast-acting drug can be life or death.
Nasal dosage forms can be delivered simply and efficiently to unconscious patients without the need of medical training; this is ever more important, due to the stressful nature of these circumstances. Nasal delivery does not require the patient to be breathing upon administration.
Examples of fast-acting commercial nasal delivered drugs are sumotryptan for migraines and Baqsimi which provides glucagon rescue for diabetics.
Conclusion
Nasal delivery is not necessarily better than injected or orally administered drugs in all cases; however, it offers a unique alternative which could be more beneficial in specific patient profiles or economic circumstances. If you’re looking for reproducible results upon application for a chronic condition, then nasal dosing may not offer advantages over other delivery routes. However, it comes into its own when trying to provide a remedy immediately and easily in emergency situations, delivering drugs to the brain whilst avoiding the blood-brain barrier and it is ideal for developing immunity within the nose a primary route for infection.
Frequently Asked Questions
What are the advantages of nasal drug delivery?
Nasal delivery offers non-invasive administration, rapid onset of action, avoidance of first-pass metabolism, and high patient compliance. It is particularly useful for delivering treatments directly to the nasal cavity for local effects, providing a fast-acting alternative in emergencies, and facilitating drug delivery to the brain by bypassing the blood-brain barrier.
How does nasal delivery help in treating neurological disorders?
The olfactory region in the nasal cavity provides a direct connection to the brain, bypassing the blood-brain barrier. This makes nasal delivery a promising method for administering therapeutics for neurological disorders, ensuring faster and more effective drug absorption.
What are the challenges of nasal drug delivery?
One of the main challenges is nasal clearance, as drugs without mucoadhesives have only 10 to 15 minutes for absorption before being removed from the nasal cavity. Additionally, ensuring the drug reaches the olfactory region for brain delivery requires specialized nasal delivery devices.
Can nasal vaccines be an alternative to injectable vaccines?
Yes, nasal vaccines offer a viable alternative to injectable vaccines. They eliminate the need for cold chain storage in the case of dry powder formulations, reduce the risk of infections from hypodermic needles, and require less training for administration.
Additional Resources
Get in touch.
If you’re looking to work with a CDMO that can support your product from preclinical development to market and beyond, then we’re here to help.





