Nasal delivery offers a number of unique opportunities for the successful delivery of drugs and vaccines either locally, systemically or directly to the central nervous system. This utility has resulted in growing interest from scientists looking to administer both therapeutic agents and vaccines via the nasal route.
In our first article, we looked at the basic anatomy of the nasal cavity and considered why it was such a good target for delivering a wide range of therapeutics and vaccines, and in our second article we discussed some of the formulation choices that need to be taken into account when developing dosage forms for the nasal route.
In this, our third and final article in the series, we examine some of the analytical techniques that are used to analyse the drug product and subsequent device performance, both for characterisation purposes and for assessing stability.
Testing Liquid or Dry Powder Devices
When considering options for nasal delivery of a drug or vaccine, the first decision to be made is which type of device to choose. Similarly, the choice of analytical methods will be very different for the two dosage form presentations, and depend greatly on the stage of development and regulatory territory.
Liquid nasal dosage forms are the most prevalent and provide the simplest and most flexible formulation approach. They also offer the option of either a unit-dose or multi-dose delivery. Analysis of the dosage form delivered by liquid devices, will include:
- Comprehensive testing of the liquid in the device (including the API and key excipients).
- Device performance testing such as droplet size, plume geometry, spray pattern and the quantity of liquid/drug delivered.
- Comprehensive testing of the dry powder drug product within the device.
- The particle size, plume geometry and quantity of dry powder delivered from the device.
Development studies
During development of nasal products, a wide range of performance attribute tests are recommended and/or stipulated by the regulatory authorities in addition to the more typical; and expected specified final product release testing.
EP guidance notes and similarly FDA detail a lot of these tests which are often dependant on both the formulation and the device and can be used to discriminate between formulation variants. These include: Delivered dose throughout the life of the product, Priming requirements, Effect of dosing orientation on emitted dose. Plume geometry and spray pattern. Another tool that has proven useful in comparing and selecting formulations during development is the transparent nasal cavity.
The effect of formulation changes, delivery device and orientation can all be visualised by use of the transparent nasal cavity. The cavity is coated such that a colour change occurs when the delivered formulation is dosed from the nasal device. By examination of the colour the specific areas of deposition in the nasal cavity can be identified and key performance failures modes such as excessive formulation run-off to the throat or excessive deposition in the nasal vestibule resulting in the formulation “dripping out” can be observed.
Testing Considerations for Phase 1 Studies
The exhaustive list of tests needed for commercial products are generally not required for first in human/Phase 1 studies, instead a more pragmatic approach can be taken. In Table 1 below are typical assays that Upperton Pharma Solutions would suggest are suitable for supporting Phase 1 studies.
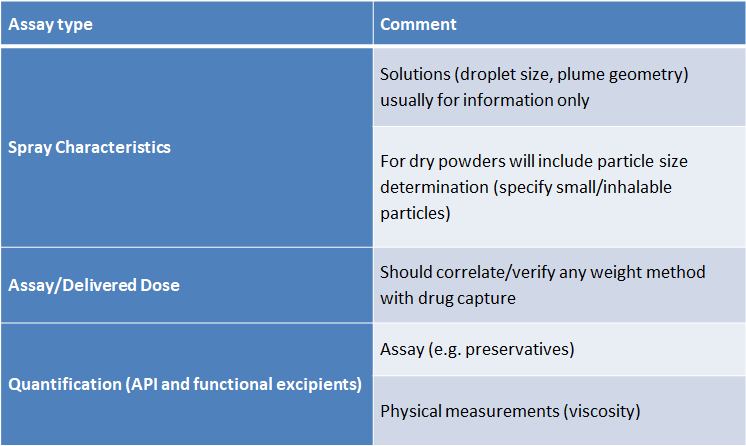
Testing Nasal Formulations: Regulatory Requirements
The degree of testing will be dictated by the regulatory setting and the stage of development of the drug/device combination. Typical testing regimens for liquid and dry powder nasal products are shown in Table 2 below:
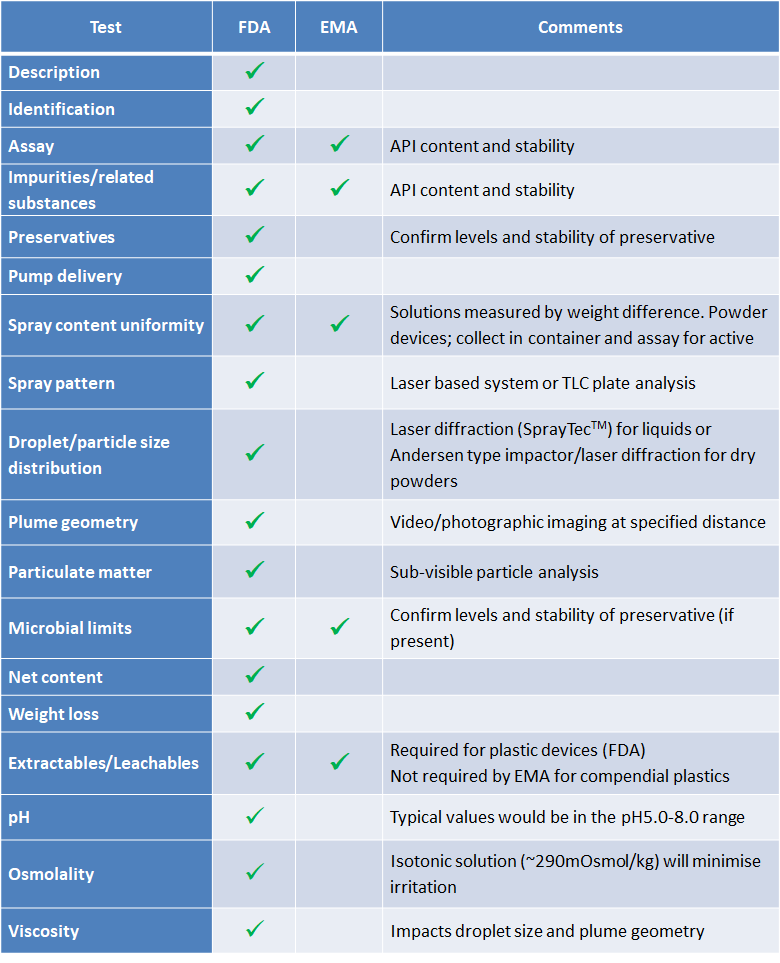
As demonstrated, there are an extensive range of tests required when developing and subsequently testing nasal dosage forms for human use. Some of these tests require sophisticated pieces of equipment, many of which might only be found in laboratories that have established nasal testing capabilities.
The range and extent of testing will mainly be dictated by the development stage of the formulation to be tested. In simple terms, products earmarked for use in early-stage clinical testing (Phase I) will require significantly less testing than products being tested for commercial use.
In early (Phase I) studies key tests will be those that impact on product safety, such as particle size/droplet size (impact on pulmonary toxicity) and emitted dose will provide key safety and delivery information to support use in first in human studies.
As the drug product moves further down the development process, the extent of testing will be required to increase to allow the drug to be sold/used commercially and more stringent tests such as device compatibility (extractables/leachables) will become necessary.
Conclusion
This series of articles has outlined the logistics and considerations associated with developing a drug/vaccine for nasal delivery. We have discussed the advantages the anatomy of the nasal cavity provides for drug delivery, the challenges of developing both liquid and dry powder formulations, and analytical techniques alongside regulatory testing requirements.
If you’ve found these articles useful, ensure to keep an eye out for future articles/newsletters from Upperton Pharma Solutions. Register for subscription or visit our archive on the Upperton website.
If you would like to discuss anything further about these articles or working with Upperton please contact us via email or call us on +44(0) 115 8557050
Get in touch.
If you’re looking to work with a CDMO that can support your product from preclinical development to market and beyond, then we’re here to help.





